Organic vs. Solid-Phase Nucleic Acid Extraction: A Comprehensive Guide for Biomedical Research and Diagnostics
This article provides a systematic comparison of organic (liquid-phase) and solid-phase nucleic acid extraction methods, crucial first steps in molecular biology and diagnostics.
Organic vs. Solid-Phase Nucleic Acid Extraction: A Comprehensive Guide for Biomedical Research and Diagnostics
Abstract
This article provides a systematic comparison of organic (liquid-phase) and solid-phase nucleic acid extraction methods, crucial first steps in molecular biology and diagnostics. Tailored for researchers, scientists, and drug development professionals, it explores the fundamental principles, biochemical mechanisms, and practical applications of each technique. The scope ranges from foundational knowledge and detailed methodologies to troubleshooting common issues and presenting validation data from recent studies. By synthesizing performance metrics, cost-benefit analyses, and suitability for various sample types and downstream applications, this review serves as a definitive guide for selecting and optimizing nucleic acid extraction protocols to enhance the reliability and efficiency of biomedical research and clinical diagnostics.
The Foundations of Nucleic Acid Extraction: Principles and Evolution of Methodologies
In the fields of molecular biology and analytical chemistry, the preparation of pure samples is a critical precursor to accurate analysis. Among the myriad of techniques available, organic extraction and solid-phase extraction (SPE) have emerged as fundamental methodologies for isolating target compounds from complex matrices [1] [2]. While both techniques aim to separate and concentrate analytes, they operate on divergent principles and offer distinct advantages and limitations. Organic extraction, often called liquid-liquid extraction, relies on the differential solubility of compounds in immiscible solvents [1]. In contrast, SPE utilizes affinity interactions between analytes and a solid sorbent material to achieve separation [2] [3]. The choice between these methods significantly impacts the efficiency, purity, and success of downstream applications, particularly in nucleic acid research and drug development. This guide provides a detailed comparative analysis of these foundational techniques, examining their core principles, historical development, and practical implementation to inform researchers in selecting the most appropriate methodology for their specific applications.
Core Principles and Mechanisms
Organic Extraction
Organic extraction is a liquid-liquid separation technique that exploits the differential solubility of biological molecules in immiscible aqueous and organic phases [1]. The fundamental principle involves partitioning biomolecules between these phases based on their chemical properties. When an organic solvent is added to an aqueous biological sample, a biphasic system forms. During centrifugation, hydrophobic molecules such as proteins and lipids migrate to the organic phase, while hydrophilic nucleic acids remain in the aqueous phase [1].
The pH of the extraction buffer is a critical factor determining selectivity. For DNA extraction, neutral to slightly basic conditions (pH 7-8) are employed to ensure DNA partitions into the aqueous phase. Conversely, acidic conditions favor RNA retention in the aqueous phase while DNA moves to the organic interphase [1]. The phenol-chloroform mixture is the most common organic combination, where phenol denatures proteins, chloroform enhances phase separation and facilitates lipid partitioning, and isoamyl alcohol is often added to reduce foam formation and stabilize the interface [1]. Following phase separation, nucleic acids in the aqueous phase are typically precipitated using ethanol or isopropanol for subsequent purification and concentration.
Solid-Phase Extraction
Solid-phase extraction operates on chromatographic principles where analytes are separated based on their specific interactions with a solid sorbent material [2] [3]. The process involves passing a liquid sample through a sorbent-packed cartridge or disk, where target compounds are retained while matrix components pass through. Retained analytes are later eluted using an appropriate solvent [2].
SPE sorbents function through several primary retention mechanisms [3]:
- Non-polar interactions: Utilizing van der Waals forces between non-polar functional groups (C18, C8, phenyl) and hydrophobic analytes from polar matrices.
- Polar interactions: Employing dipole-dipole or hydrogen bonding with polar functional groups (diol, amino, cyano) for polar analyte retention from non-polar matrices.
- Ion-exchange: Leveraging electrostatic interactions between charged analytes and oppositely charged sorbent functional groups (cationic or anionic).
- Mixed-mode: Combining multiple mechanisms, typically hydrophobic and ion-exchange, for highly selective separations [3].
The SPE process follows a systematic sequence: sorbent conditioning to activate functional groups, sample loading, washing to remove impurities, and elution of purified analytes [2] [3]. This multi-step approach enables significant sample cleanup and concentration, making it particularly valuable for complex matrices.
Table 1: Fundamental Characteristics of Extraction Techniques
| Parameter | Organic Extraction | Solid-Phase Extraction |
|---|---|---|
| Primary Principle | Differential solubility partitioning | Affinity adsorption chromatography |
| Phase System | Liquid-Liquid | Solid-Liquid |
| Key Mechanisms | Hydrophobicity, solubility | Van der Waals, hydrogen bonding, ionic interactions |
| Critical Factors | pH, solvent polarity, centrifugation | Sorbent chemistry, solvent polarity, pH |
| Typical Format | Centrifuge tubes | Cartridges, disks, pipette tips |
| Phase Separation | Physical separation after centrifugation | Selective elution from solid matrix |
Historical Context and Development
Evolution of Organic Extraction
The development of organic extraction methodologies parallels advances in molecular biology throughout the 20th century. The technique gained prominence with the phenol-chloroform method becoming a standard laboratory procedure for nucleic acid purification [1]. This method established itself as a reliable approach for obtaining high-purity DNA and RNA, particularly for molecular cloning and early genomic studies. The introduction of specialized reagents like TRIzol, which combines phenol and guanidine thiocyanate in a monophasic solution, streamlined the simultaneous isolation of RNA, DNA, and proteins from a single sample [1]. Despite the emergence of newer technologies, organic extraction remains valued for its effectiveness with challenging samples and cost-efficiency for processing large volumes.
Development of Solid-Phase Extraction
SPE has a more diverse technological evolution, with its first analytical applications emerging in the 1940s-1950s for analyzing organic traces in water samples [4]. Animal charcoal served as the earliest adsorbent for removing pigments from reaction mixtures [2]. The technique progressed through several distinct developmental phases [2] [4]:
The Age of Active Carbon (1950s-1960s): Early applications primarily utilized activated carbon for concentrating organic pollutants from water, though recovery was often problematic due to strong adsorption [4].
The Search for Appropriate Materials (1970s-1980s): Researchers investigated various synthetic polymers, including styrenedivinylbenzene resins, to overcome the limitations of carbon [2] [4]. The introduction of octadecyl silica (C18) bonded phases in the late 1970s marked a significant advancement, enabling more predictable reversed-phase interactions [2].
The Age of Technical Developments (1980s): This period saw the commercialization of pre-packed columns and cartridges, standardization of protocols, and theoretical modeling of SPE processes [4]. The introduction of SPE disks and membranes in 1989 provided greater cross-sectional areas for processing large sample volumes more efficiently [2].
Modern SPE continues to evolve with developments in monolithic sorbents, molecularly imprinted polymers, and various configurations including pipette-tip SPE for small volumes and 96-well plates for high-throughput applications [2].
Table 2: Historical Timeline of Extraction Method Development
| Time Period | Organic Extraction Milestones | Solid-Phase Extraction Milestones |
|---|---|---|
| 1940s-1950s | Early phenol-based methods | First SPE applications with active carbon |
| 1960s-1970s | Standardization of phenol-chloroform protocols | Development of synthetic polymer sorbents |
| 1970s-1980s | Adoption for molecular cloning | Introduction of bonded silica phases (C18) |
| 1980s-1990s | TRIzol and commercial reagent systems | Commercial pre-packed columns; SPE disks |
| 1990s-Present | Automation and safety improvements | Monolithic sorbents; high-throughput formats |
Comparative Performance Analysis
Quantitative Method Comparison
Evaluating the performance characteristics of organic extraction and SPE reveals a clear trade-off between purity, efficiency, and practicality. The following table summarizes key performance metrics based on experimental data and technical specifications:
Table 3: Performance Comparison of Organic vs. Solid-Phase Extraction
| Performance Parameter | Organic Extraction | Solid-Phase Extraction | Experimental Measurement Context |
|---|---|---|---|
| Protein Removal Efficiency | High (effective denaturation) | Variable (depends on sorbent) | Protein quantification post-extraction; SDS-PAGE |
| Nucleic Acid Yield | High for complex samples | High to moderate | Spectrophotometry (A260)/fluorometry |
| Typical Processing Time | 30-90 minutes | 15-45 minutes | Hands-on time for standard protocols |
| Detection Limits | Higher (ng/mL range) | Lower (0.1-1.0 ng/mL for SPME) | Analytical instrument detection limits [5] |
| Sample Volume Capacity | Flexible (μL to mL) | Cartridge-dependent (500μL-50mL) | Manufacturer specifications [2] |
| Risk of Emulsion Formation | Moderate to High | None | Observation during method development |
| Cross-Contamination Risk | Moderate (phase mixing) | Low with proper washing | PCR amplification of non-target sequences |
| Solvent Consumption | High (multiple volumes) | Low (focused elution) | Solvent volume per extraction |
Applications in Nucleic Acid Research
Both techniques have found specific niches in nucleic acid extraction workflows, each offering distinct advantages for particular applications:
Organic Extraction Applications:
- Genomic DNA extraction from complex tissues and biological samples [1]
- RNA purification for transcriptomic studies and RNA-seq applications [1]
- Plasmid isolation when high purity is required for cloning [1]
- Challenging samples with high lipid content or abundant proteins
Solid-Phase Extraction Applications:
- High-throughput processing using 96-well plate formats [2]
- Automated extraction systems for clinical and diagnostic testing [1]
- Environmental DNA analysis from water samples [4] [6]
- Forensic applications where sample integrity is critical [5]
- Selective isolation of specific nucleic acid types using functionalized sorbents
Experimental Protocols
Standard Organic Extraction Protocol for DNA
This protocol outlines the phenol-chloroform extraction method for DNA purification from biological samples [1]:
Reagents and Materials:
- Phenol:chloroform:isoamyl alcohol (25:24:1 ratio)
- Cell lysis buffer (e.g., containing SDS and proteinase K)
- Chloroform alone
- Ethanol (70% and absolute)
- Isopropanol
- TE buffer or nuclease-free water
- Microcentrifuge tubes
- Refrigerated microcentrifuge
Methodology:
- Cell Lysis: Homogenize tissue or cell sample in appropriate lysis buffer. Incubate with proteinase K (if required) at 50-65°C until completely lysed.
- First Extraction: Add an equal volume of phenol:chloroform:isoamyl alcohol to the lysate. Mix thoroughly by inversion for 2-3 minutes. Centrifuge at 12,000 × g for 5 minutes at room temperature.
- Aqueous Phase Recovery: Carefully transfer the upper aqueous phase to a fresh tube, avoiding the interphase and organic layer.
- Second Extraction: Add an equal volume of chloroform to the aqueous phase. Mix thoroughly and centrifuge as before.
- DNA Precipitation: Transfer the aqueous phase to a new tube. Add 0.5-1 volume of isopropanol or 2 volumes of cold ethanol. Mix by inversion until DNA precipitates.
- DNA Recovery: Centrifuge at 12,000 × g for 10 minutes. Carefully decant the supernatant.
- DNA Washing: Add 1 mL of 70% ethanol. Centrifuge at 12,000 × g for 5 minutes. Carefully decant the ethanol.
- DNA Resuspension: Air-dry the pellet for 5-10 minutes (do not overdry). Resuspend in TE buffer or nuclease-free water.
Critical Steps:
- Maintain neutral pH (7-8) for DNA extraction
- Avoid transferring any organic phase during aqueous phase recovery
- Use wide-bore tips for pipetting high molecular weight DNA
- Ensure complete resuspension of the DNA pellet
Standard Solid-Phase Extraction Protocol for Nucleic Acids
This protocol describes SPE using silica-based membranes for nucleic acid purification [2] [3]:
Reagents and Materials:
- Silica-based SPE cartridge or spin column
- Cell lysis buffer (e.g., guanidine hydrochloride)
- Wash buffers (typically ethanol-based)
- Elution buffer (TE or nuclease-free water)
- Vacuum manifold or centrifuge
- Collection tubes
Methodology:
- Sorbent Conditioning: Apply 1-2 column volumes of conditioning solvent (e.g., methanol) to the sorbent bed. Follow with 1-2 column volumes of equilibration buffer (typically water or low-salt buffer).
- Sample Loading: Adjust sample conditions (e.g., add chaotropic salts) to promote nucleic acid binding. Apply sample to the conditioned sorbent under controlled flow rates (1-2 mL/min).
- Washing: Apply 2-3 column volumes of wash buffer (typically ethanol-based) to remove impurities. Ensure the sorbent does not dry completely between steps.
- Elution: Apply 1-2 column volumes of pre-warmed (65°C) elution buffer to the sorbent. Allow it to incubate for 1-2 minutes before applying pressure or centrifugation.
- Storage: Collect eluate containing purified nucleic acids. Store at appropriate temperatures for downstream applications.
Critical Steps:
- Do not let sorbent dry out before elution step
- Optimize flow rates for binding and washing steps
- Use pre-warmed elution buffer for higher yields
- Select appropriate sorbent chemistry for target nucleic acids
Workflow Visualization
Diagram 1: Comparative Workflows of Organic and Solid-Phase Extraction Methods
Research Reagent Solutions
Table 4: Essential Reagents and Materials for Extraction Protocols
| Reagent/Material | Primary Function | Application in Organic Extraction | Application in SPE |
|---|---|---|---|
| Phenol | Protein denaturation | Essential for protein removal | Not typically used |
| Chloroform | Lipid solubilization, phase separation | Enhances protein partitioning | Not typically used |
| Isoamyl Alcohol | Foam reduction | Prevents emulsion formation | Not typically used |
| Silica Sorbents | Nucleic acid binding | Not typically used | Primary retention matrix |
| Chaotropic Salts | Nucleic acid binding promotion | Optional for precipitation | Essential for binding to silica |
| C18 Bonded Phase | Hydrophobic interactions | Not applicable | Reversed-phase extraction |
| Ion-Exchange Resins | Ionic interactions | Not applicable | Charged analyte retention |
| Ethanol/Isopropanol | Nucleic acid precipitation | Essential for precipitation | Wash buffer component |
| Guanidine Hydrochloride | Protein denaturation, nuclease inhibition | Optional in lysis buffers | Common in binding buffers |
| TE Buffer | Nucleic acid storage and elution | Resuspension buffer | Primary elution solution |
Organic extraction and solid-phase extraction represent two fundamentally different approaches to sample preparation, each with distinctive strengths and limitations. Organic extraction remains the gold standard for achieving high-purity nucleic acids from challenging samples, leveraging the proven efficacy of liquid-liquid partitioning [1]. Its disadvantages include labor-intensive procedures, use of hazardous solvents, and difficulty in automation. In contrast, SPE technologies offer faster processing, reduced solvent consumption, and excellent compatibility with automation and high-throughput workflows [2] [3].
The choice between these methodologies depends on multiple factors: the nature of the starting material, required purity levels, throughput needs, available resources, and safety considerations. For laboratories processing diverse sample types with varying requirements, maintaining expertise in both techniques provides the flexibility needed to address the broad spectrum of challenges encountered in modern nucleic acid research. As both technologies continue to evolve—with organic extraction focusing on safety improvements and SPE advancing through novel sorbent chemistries—researchers can expect continued enhancement in the efficiency and effectiveness of these fundamental sample preparation tools.
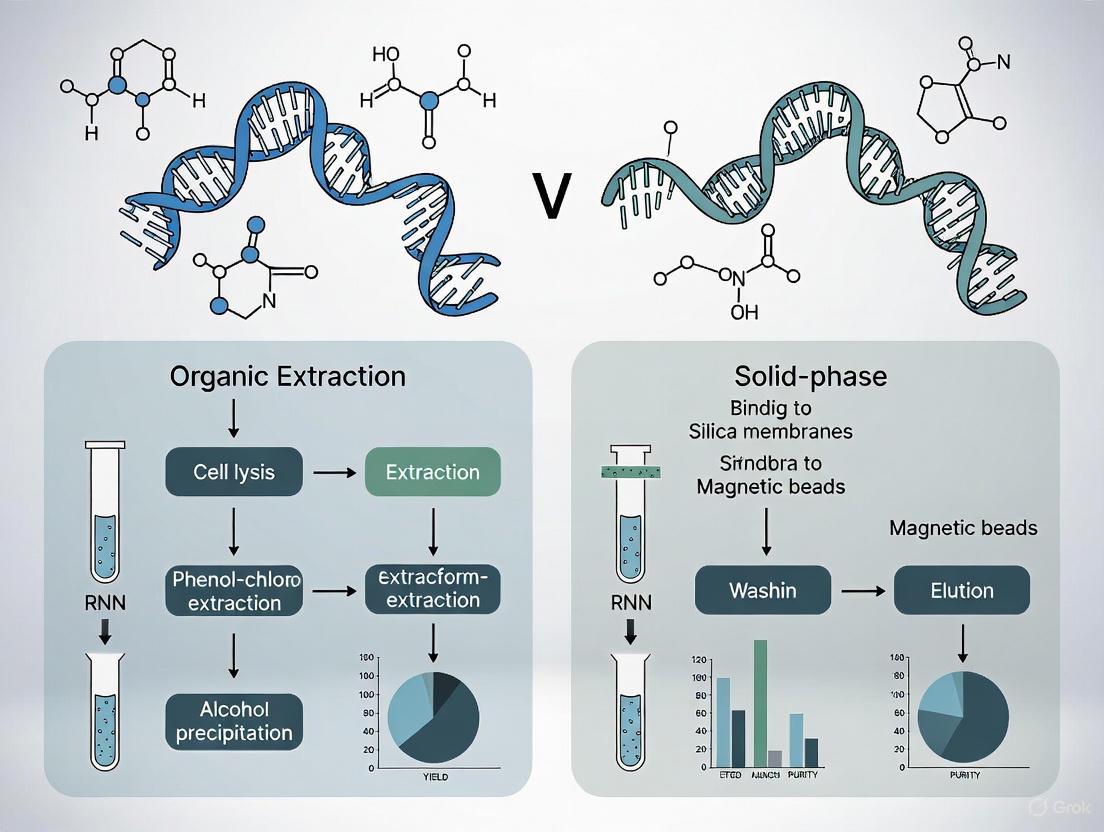
Nucleic acid extraction is a foundational technique in molecular biology, serving as a critical pre-analytical step for a vast array of applications from clinical diagnostics to advanced research. The efficacy of downstream processes, including PCR, sequencing, and microarray analysis, is profoundly dependent on the quality, purity, and yield of the isolated nucleic acids. The core principles of extraction, whether applied to DNA or RNA, revolve around four fundamental steps: lysis to disrupt cells and release nucleic acids, binding of the nucleic acids to a solid or liquid matrix, purification by removing contaminants and inhibitors, and concentration of the target nucleic acids into a small, usable volume. This guide delves into the specifics of these steps, providing a detailed comparison between the two predominant methodological philosophies: organic extraction and solid-phase extraction. By framing this within a broader thesis on extraction research, we will objectively compare product performance using supporting experimental data to inform the choices of researchers, scientists, and drug development professionals.
The Core Principles: Four Fundamental Steps Explained
All nucleic acid extraction protocols, regardless of their specific chemistry, are built upon four essential stages. The following diagram illustrates the logical workflow and the key decisions at each step.
Step 1: Lysis
The first step involves breaking open the cell and nuclear membranes to release the nucleic acids into solution. Lysis must be efficient enough to access the genetic material without causing excessive degradation.
- Methods: Lysis can be achieved through chemical means (e.g., detergents, chaotropic salts, and enzymes like proteinase K) or mechanical means (e.g., bead-beating or homogenization) [7] [8]. The choice is sample-dependent; for instance, gram-positive bacteria or plant tissues with robust cell walls often require vigorous mechanical disruption in addition to chemical lysis [9] [8]. Chaotropic salts like guanidinium isothiocyanate not only aid in lysis but also denature proteins and protect nucleic acids from nucleases [10].
Step 2: Binding
Once released, the nucleic acids must be captured and separated from the lysate. This is the stage where the primary distinction between extraction methods emerges.
- Solid-Phase Binding: This method relies on the affinity of nucleic acids for a solid surface under specific conditions. In the presence of chaotropic salts, the negatively charged phosphate backbone of DNA and RNA binds to a silica matrix [7] [10]. This matrix can be formatted as a spin column membrane or as silica-coated magnetic beads.
- Liquid-Phase (Organic) Binding: In organic extraction, the lysate is mixed with phenol-chloroform. When centrifuged, the solution separates into an organic phase (containing denatured proteins and lipids), an interphase, and an aqueous phase where the nucleic acids reside [7] [11]. The binding here is not to a solid phase but a partition into the aqueous phase based on solubility.
Step 3: Purification (Washing)
The bound nucleic acids are associated with contaminants like proteins, salts, and other cellular debris that can inhibit downstream applications. The purification step removes these impurities.
- Solid-Phase Purification: The silica matrix is washed multiple times with ethanol-based buffers containing detergents or alcohols to remove contaminants while leaving the nucleic acids bound [7] [10].
- Liquid-Phase Purification: The aqueous phase containing the nucleic acids is carefully extracted from the phenol-chloroform mixture, physically separating it from the denatured proteins in the organic phase [11]. Further purification may involve additional chloroform extractions to remove residual phenol.
Step 4: Elution and Concentration
The final step is to recover the purified nucleic acids in a concentrated form suitable for analysis.
- Solid-Phase Elution: The nucleic acids are released from the silica matrix using a low-ionic-strength buffer like TE or nuclease-free water. The small volume of the elution buffer ensures the nucleic acids are concentrated [7] [12].
- Liquid-Phase Concentration: Nucleic acids in the aqueous phase are concentrated by precipitation, typically using isopropanol or ethanol in the presence of a salt like sodium acetate. The precipitated nucleic acid pellet is then washed with ethanol to remove residual salt and rehydrated in a small volume of buffer [7] [11].
Methodologies Compared: Organic vs. Solid-Phase Extraction
The choice between organic and solid-phase methods fundamentally shapes the extraction workflow, performance, and applicability. The table below summarizes their core characteristics.
Table 1: Fundamental Comparison of Organic and Solid-Phase Extraction Methods
| Feature | Organic Extraction | Solid-Phase Extraction |
|---|---|---|
| Core Principle | Liquid-liquid partitioning using phenol-chloroform [7] [11] | Binding to a solid silica matrix (columns or magnetic beads) [7] [10] |
| Typical Yield | High | Variable; can be very high with optimized methods (e.g., SHIFT-SP) [12] [10] |
| Purity | High, effective protein removal [11] | High, though may require thorough washing to remove chaotropes [10] |
| Throughput | Low, manual and labor-intensive [11] | High, easily automated [7] [13] |
| Automation | Difficult to automate [11] | Highly amenable to automation (liquid handlers, KingFisher systems) [13] [9] |
| Key Advantage | Considered a "gold standard"; effective on tough samples [11] | Safety, speed, and suitability for high-throughput workflows [7] |
| Key Disadvantage | Use of toxic, hazardous chemicals [7] [11] | Potential for low yield if binding is inefficient [11] |
Evolution of Solid-Phase Methods: Silica Columns and Magnetic Beads
Solid-phase extraction has become the dominant approach, particularly in clinical and high-throughput settings, and has evolved into two main formats.
Silica Spin Columns: In this format, the silica is embedded in a membrane within a plastic column. The lysate is passed through the membrane by centrifugation or vacuum, facilitating binding, washing, and elution in a self-contained unit [7] [11]. A limitation is that viscous samples or overloading can clog the membrane [11].
Magnetic Bead Technology: This method uses silica-coated paramagnetic beads. When an external magnetic field is applied, the beads (with bound nucleic acids) are immobilized, allowing the supernatant to be easily removed and exchanged for wash and elution buffers without centrifugation or vacuum filtration [7] [9]. This makes the process non-clogging and exceptionally well-suited for full automation [7] [9]. Recent research has focused on optimizing magnetic bead protocols to maximize speed and yield, as demonstrated by the SHIFT-SP method, which achieves extraction in 6-7 minutes with near-complete nucleic acid recovery [12] [10].
Experimental Data and Performance Comparison
Theoretical advantages must be validated with experimental data. The following tables and experimental overviews provide a direct, objective comparison of extraction methodologies and systems.
Direct Method Comparison: Yield and Time
A 2025 study developed and benchmarked the SHIFT-SP method, a magnetic silica bead-based protocol, against other common commercial techniques, providing clear quantitative data on yield and processing time [12] [10].
Table 2: Performance Comparison of Extraction Methods from a 2025 Study [12] [10]
| Extraction Method | Total Processing Time | Relative DNA Yield | Key Characteristics |
|---|---|---|---|
| SHIFT-SP (Magnetic Bead) | 6 - 7 minutes | ~100% | Optimized pH and tip-based mixing; automation-compatible. |
| Commercial Bead-Based | ~40 minutes | ~100% | Similar yield but significantly longer processing time. |
| Commercial Column-Based | ~25 minutes | ~50% | Faster than standard bead methods but half the yield. |
Experimental Protocol Summary [10]: The study optimized binding and elution conditions for a magnetic silica bead workflow. Key parameters included:
- Binding Buffer pH: Systematically compared pH 4.1 vs. 8.6, finding that a lower pH (4.1) reduced electrostatic repulsion between silica and DNA, increasing binding efficiency to 98.2% within 10 minutes.
- Mixing Mode: Compared orbital shaking to a pipette "tip-based" method (repeated aspiration/dispersion). Tip-based mixing achieved ~85% binding in 1 minute, a efficiency level that took 5 minutes with orbital shaking.
- Elution: Optimized temperature, duration, and buffer pH to maximize the recovery of bound nucleic acids.
Comparison of Automated Extraction Systems
Automation is key for standardizing high-throughput workflows. A 2024 study compared three automated nucleic acid extractors for processing complex human stool samples, a challenging matrix with diverse inhibitors and microbial cell types [9].
Table 3: Evaluation of Automated Nucleic Acid Extraction Systems for Stool Samples [9]
| Extraction System | Technology Basis | Key Findings (Stool Samples) |
|---|---|---|
| KingFisher Apex | Magnetic bead-based | Effective recovery of Gram-positive bacteria; low inter-sample variability. |
| Maxwell RSC 16 | Magnetic bead-based / Cartridge-based | Robust performance; integrated system with pre-filled cartridges. |
| GenePure Pro | Magnetic bead-based | Comparable performance; differences in yield and variability observed. |
| All Automated Systems | - | Critical Finding: The inclusion of a bead-beating (mechanical lysis) step prior to automated extraction was essential for the effective lysis of Gram-positive bacteria and yielded a more representative microbial profile, regardless of the instrument used. |
Experimental Protocol Summary [9]: The study used triplicate fecal aliquots from healthy volunteers and a mock microbial community standard.
- Mechanical Lysis: Samples were homogenized using a FastPrep-24 bead-beating grinder at 6.0 m/s for 40 seconds.
- DNA Extraction & Quantification: Automated extraction was performed on each system according to manufacturer protocols. DNA was quantified using a Qubit fluorometer (for concentration) and a NanoDrop spectrophotometer (for purity).
- Downstream Analysis: 16S rRNA amplicon sequencing was performed to assess the impact of the extraction method on microbial community profiles (alpha- and beta-diversity).
The Scientist's Toolkit: Essential Research Reagent Solutions
Selecting the appropriate reagents and kits is critical for success. The following table details key solutions used in the featured experiments and the broader field.
Table 4: Essential Reagents and Kits for Nucleic Acid Extraction
| Item / Kit Name | Function / Application | Specific Example from Research |
|---|---|---|
| Phenol-Chloroform | Organic extraction; partitions nucleic acids into aqueous phase while denaturing and removing proteins [7] [11]. | A standard, well-established protocol for high-purity isolation, though hazardous [11]. |
| Silica Spin Columns | Solid-phase extraction; membrane binds nucleic acids for in-column washing and elution [7]. | Used in commercial column-based kits compared in the 2025 study [10]. |
| Magnetic Silica Beads | Solid-phase extraction; beads bind nucleic acids for magnetic separation, enabling automation [7] [9]. | Core component of the SHIFT-SP method [10] and automated systems like KingFisher Apex and Maxwell RSC [9]. |
| Chaotropic Salts (e.g., Guanidine HCl) | Denature proteins, inactivate nucleases, and create conditions for nucleic acid binding to silica [7] [10]. | Key component of the Lysis Binding Buffer (LBB) in the VERSANT and SHIFT-SP protocols [10]. |
| Proteinase K | Enzymatic lysis; digests proteins and helps to disrupt cellular structures [8]. | Commonly used in tissue lysis protocols to improve yield and purity [8]. |
| DNA/RNA Shield | Sample stabilization; prevents nucleic acid degradation during sample storage and transport [9]. | Used in the stool sample study to preserve integrity before extraction [9]. |
Practical Application and Workflow Selection
Translating experimental data into a practical laboratory decision requires considering sample type, downstream application, and operational needs. The following diagram outlines a decision pathway for selecting an extraction method.
Sample Type-Specific Considerations
- Challenging Matrices: For samples with complex compositions like stool, plants, or forensic swabs, specialized kits are essential. These kits often include additives like polyvinylpyrrolidone (PVP) to bind polyphenols from plants or enhanced wash buffers to remove PCR inhibitors like bile salts from stool [14] [8].
- Stabilized Samples: Samples stored in stabilization media (e.g., saliva, stool swabs) may yield less DNA per volume than raw samples, necessitating potential adjustments to input volume to meet the requirements of downstream assays [8].
- Forensic and Low-Input Samples: For challenging forensic applications like body fluid identification from saliva, the choice of extraction kit significantly impacts miRNA recovery and detection sensitivity, which is crucial for downstream RT-qPCR analysis [15]. Furthermore, high-yield methods like SHIFT-SP are particularly beneficial for low-concentration samples, such as microbes in enriched whole blood, enabling successful whole genome amplification and sequencing [10].
The fundamental steps of lysis, binding, purification, and elution/concentration form the unchanging backbone of nucleic acid extraction. However, the methodologies to execute these steps are constantly evolving. While organic extraction remains a powerful, gold-standard method for its purity and effectiveness on challenging samples, the trends in research and clinical practice are decisively shifting towards solid-phase techniques, particularly magnetic bead-based automation. The driving forces are clear: enhanced safety, greater throughput, reduced hands-on time, and improved reproducibility. As evidenced by recent studies, the optimization of solid-phase protocols is closing historical performance gaps, achieving speeds and yields that were previously unattainable. For the modern researcher, the optimal choice is not a matter of declaring one method the universal winner, but of strategically matching the method—be it organic, column-based, or magnetic bead-based—to the specific constraints of the sample, the application, and the operational environment.
This guide provides an objective comparison of nucleic acid isolation methods, focusing on the biochemical principles of chaotropic salt, pH, and silica interface techniques. Designed for researchers and drug development professionals, it synthesizes experimental data to evaluate performance across key parameters including yield, purity, time, and cost, framed within the broader research context of organic versus solid-phase extraction methodologies.
Nucleic acid isolation relies on sophisticated biochemical interactions between chaotropic salts, silica surfaces, and nucleic acids at specific pH levels. Chaotropic salts (e.g., guanidine hydrochloride (GuHCl) and guanidine thiocyanate) function by disrupting the hydrogen-bonded network of water molecules, thereby solubilizing hydrophobic molecules and denaturing proteins that would otherwise interfere with nucleic acid purification. [16] [17] In the presence of these salts, nucleic acids lose their hydration shell and become susceptible to binding onto solid surfaces. The silica interface mechanism involves the adsorption of the negatively charged phosphate backbone of nucleic acids onto the positively charged silica surface, a process mediated by chaotropic salts acting as a cation bridge. [16] [18] The pH of the binding environment critically influences this interaction; a lower pH (e.g., pH 4.1) reduces the negative charge on both silica and DNA, minimizing electrostatic repulsion and significantly enhancing binding efficiency compared to neutral or alkaline conditions. [10] Understanding these intertwined mechanisms is essential for selecting and optimizing extraction methods for specific downstream applications.
Comparative Analysis of Extraction Methodologies
The following analysis compares three primary extraction methodologies: chaotropic salt/silica-based, magnetic nanoparticle, and organic extraction.
Performance Comparison Table
Table: Quantitative Comparison of Nucleic Acid Extraction Method Performance
| Extraction Method | Reported DNA Yield | Extraction Time | Cost per Sample (USD) | Suitability for Complex Samples | Key Advantages |
|---|---|---|---|---|---|
| Chaotropic Salts + Silica (SHIFT-SP) [10] | ~96% (1000 ng input) | 6-7 minutes | Medium (Kit-dependent) | High (Optimized for stool, blood) [16] [10] | High speed, high yield, automation-compatible |
| Magnetic Ionic Liquids (MILs) [18] | High (qPCR/LAMP compatible) | < 30 minutes | Low (Green synthesis) | High (Milk, plasma, plant tissue) [18] | Direct integration with amplification, minimal purification |
| Chelex 100 Resin [19] | Highest yield in comparison study | ~90 minutes | Very Low (33x cheaper than kits) [19] | Medium (Nasopharyngeal samples) [19] | Extreme cost-effectiveness, simple protocol, high yield |
| Phenol-Chloroform (Organic) [19] | Lower yield in comparison study | >2 hours | Low (13x cheaper than kits) [19] | High | Effective inhibitor removal, no size bias |
| Traditional Silica Columns [10] [20] | ~50% of input DNA [10] | ~25 minutes [10] | High [19] | Medium to High | Widespread use, good purity |
| Magnetic Nanoparticles (MNPs) [21] | High quality and quantity [21] | ~40 minutes [10] | Very Low [21] | High (Bacterial cells, blood serum) [21] | Cost-effective, easy manipulation, amenable to automation |
- Chaotropic Salt and Silica Optimization: A study optimizing a magnetic silica bead-based method (SHIFT-SP) demonstrated that binding buffer pH drastically affects efficiency. At pH 4.1, 98.2% of input DNA was bound to beads within 10 minutes, whereas only 84.3% was bound at pH 8.6. [10] The mode of bead mixing was also critical; a "tip-based" method achieved ~85% DNA binding in 1 minute, a significant improvement over orbital shaking. [10]
- Magnetic Ionic Liquids (MILs): MILs like [Ni(OIm)62+][NTf2-]2 interact with DNA via electrostatic, π-π stacking, and van der Waals forces. [18] They enable direct DNA extraction from complex matrices such as diluted human plasma and vitamin D milk, with subsequent integration into qPCR or LAMP assays without the need for separate DNA elution steps. One study detected E. coli at concentrations as low as 5.2 CFU mL⁻¹ in milk using a MIL-LAMP assay. [18]
- Cost and Efficiency of Alternative Methods: A comprehensive cost-analysis revealed that in-house MNP-based protocols can cost less than $0.19 per sample, compared to ~$1.34 for a commercial MNP-kit and ~$13.37 for a column-based kit. [21] Another independent comparison found the Chelex 100 method to be 33 times cheaper than a commercial QIAamp kit and 13 times cheaper than phenol-chloroform, while also providing the highest DNA yield. [19]
- Inhibitor Challenges with Silica Columns: Research indicates that some commercial silica columns can elute an unidentified substance that inhibits downstream enzymatic reactions like sequencing and digestion if the eluted DNA is not sufficiently diluted. [20] This highlights a potential limitation of some commercial solid-phase methods that may not be apparent in standard yield and purity measurements.
Detailed Experimental Protocols
This protocol is designed for the detection of Helicobacter pylori and antibiotic resistance markers from stool specimens.
- Lysis Buffer: 2.5 M guanidine hydrochloride (GuHCl), 1% SDS, 40 mM Tris-HCl, 10 mM EDTA, 0.5% Triton X-100, pH 8.0.
- Procedure:
- Lysis: Add 200 μL of stool sample to 500 μL of lysis buffer and 40 μL of proteinase K (20 mg/mL). Vortex and incubate at 65°C for 15 minutes.
- Binding: Add 200 μL of 100% isopropanol and 20 μL of silicon-hydroxyl magnetic beads (Si-OH MBs) to the lysate. Mix thoroughly and incubate at room temperature for 10 minutes.
- Washing: Separate the beads on a magnetic rack and discard the supernatant.
- Wash 1: Wash with 700 μL of a solution containing 2.5 M GuHCl and 40% ethanol.
- Wash 2: Wash with 700 μL of 80% ethanol.
- Elution: Air-dry the magnetic beads and elute the purified nucleic acids in 50 μL of nuclease-free water.
- Key Parameters: The optimal concentration of GuHCl was found to be 2.5 M, and SDS at 1% provided the most efficient lysis and highest qPCR sensitivity. [16]
This novel method simultaneously removes proteins and precipitates DNA, addressing enzymatic inhibition from silica columns.
- Reagents: Chaotropic salt (e.g., 2.7 M Guanidine Hydrochloride or 1 M Guanidine Thiocyanate), precipitation agent (50% Isopropanol or 20% PEG-8000), and a protein dilution solution.
- Procedure:
- Precipitation: To the sample, add one volume of chaotropic salt solution and one volume of precipitation agent (e.g., isopropanol). Mix gently and incubate at room temperature for 2 minutes.
- Protein Dilution: Add one volume of protein dilution solution to further deactivate any residual proteins.
- Pellet Washing: Centrifuge to pellet the DNA. Carefully remove the supernatant and wash the pellet with 70% ethanol to remove residual salts.
- Resuspension: Air-dry the pellet and resuspend the DNA in water or TE buffer.
- Key Findings: This one-step precipitation method effectively eliminated proteinase K activity to undetectable levels and avoided the enzymatic inhibition observed with concentrated silica-column eluents. [20]
Workflow and Mechanism Visualization
Chaotropic Salt and Silica-Binding Mechanism
The following diagram illustrates the key steps and biochemical environment involved in nucleic acid binding to silica in the presence of chaotropic salts.
Comparison of Major Extraction Workflows
This diagram contrasts the procedural steps and time investment of three primary extraction methodologies.
The Scientist's Toolkit: Key Research Reagents
Table: Essential Reagents for Nucleic Acid Extraction and Their Functions
| Reagent | Biochemical Function | Typical Working Concentration | Key Consideration |
|---|---|---|---|
| Guanidine Hydrochloride (GuHCl) | Chaotropic agent; denatures proteins, facilitates NA binding to silica [16] [17] | 2.5 - 4 M [16] [20] | Concentration affects lysis efficiency and PCR compatibility [16] |
| Sodium Dodecyl Sulfate (SDS) | Ionic detergent; disrupts lipid membranes and solubilizes proteins [16] | 1% (w/v) [16] | Can inhibit PCR if not thoroughly removed [18] |
| Silica-coated Magnetic Beads | Solid-phase matrix for NA binding; enables magnetic separation [16] [10] | Varies by bead size/surface area | Binding efficiency is highly dependent on pH and mixing mode [10] |
| Sodium Acetate (NaOAc) | Source of cations (Na+); neutralizes DNA charge to facilitate alcohol precipitation [19] | 0.3 M (final concentration) | Standard for ethanol precipitation steps |
| Proteinase K | Broad-spectrum serine protease; degrades nucleases and cellular proteins [19] | 0.2 - 1 mg/mL [16] [19] | Requires incubation at 56°C for optimal activity |
| Chelex 100 Resin | Chelating polymer; binds metal ions to inactivate nucleases [19] | 5-10% (w/v) suspension [19] | Fast, low-cost method; suitable for PCR but not for long-term DNA storage [19] |
| Magnetic Ionic Liquids (MILs) | Paramagnetic solvents; extract NA via multiple interactions, compatible with direct amplification [18] | Solvent phase in extraction | Enables direct integration with LAMP/qPCR, reducing hands-on time [18] |
Nucleic acid extraction is a foundational technique in molecular biology, serving as the critical first step for a vast array of applications from basic research to clinical diagnostics. The methods for isolating DNA and RNA have evolved significantly, driven by the competing demands for yield, purity, speed, safety, and scalability. This evolution represents a broad thesis in life sciences methodology: the transition from classic organic, solution-based chemistry to sophisticated solid-phase protocols. This guide objectively charts this historical progression, comparing the performance of phenol-chloroform extraction, silica spin columns, and magnetic beads through the lens of published experimental data to inform researchers and drug development professionals in their selection of purification technologies.
The Evolution of Extraction Technologies
The following timeline illustrates the key milestones in the development of mainstream nucleic acid extraction methods:
The core principle of nucleic acid extraction involves three key steps: cell disruption (lysis), separation/purification of nucleic acids from other cellular components, and concentration of the purified nucleic acids [22]. The methods discussed here diverge fundamentally in how they achieve the critical purification step.
Phenol-Chloroform Extraction (Organic Phase Separation)
This traditional method relies on liquid-phase separation to purify nucleic acids [23] [24]. In this process, a sample is mixed with TRIzol (containing phenol and guanidinium salts), which lyses cells and inactivates nucleases. The subsequent addition of chloroform and centrifugation creates distinct phases: RNA remains in the aqueous phase, proteins in the organic phase, and DNA at the interphase [23]. The desired nucleic acid is then carefully pipetted and concentrated via ethanol precipitation.
Silica Spin Column (Solid-Phase Extraction)
This method utilizes a silica membrane in a column format to bind nucleic acids in the presence of chaotropic salts [23] [22]. The sample is first lysed, and the lysate is mixed with ethanol or isopropanol before being loaded onto the column. Under high-salt conditions, the negatively charged nucleic acid backbone binds to the silica membrane, while contaminants pass through. The membrane is washed, and pure nucleic acids are eluted in a low-salt buffer or water.
Magnetic Beads (Solid-Phase Extraction)
This is a modification of solid-phase extraction where silica-coated paramagnetic beads replace the column [23] [24]. After sample lysis, the magnetic beads are added, and nucleic acids bind to them in the presence of chaotropic salts and alcohol. A magnet is used to immobilize the beads, allowing the supernatant to be removed. The bead-bound nucleic acids are washed and then eluted.
Comparative Performance Data from Experimental Studies
Yield and Purity Across Sample Types
Table 1: Comparison of RNA Yield and Purity from Blood and Oral Swab Samples [25]
| Sample Type | Extraction Method | Average Yield (ng/μL) | Average Purity (A260/A280) |
|---|---|---|---|
| Blood | Modified Manual AGPC | Significantly Higher | Significantly Lower |
| Blood | QIAamp Viral RNA Mini Kit | Lower | Significantly Higher |
| Blood | OxGEn RNA Kit | Lower | Significantly Higher |
| Oral Swab | Modified Manual AGPC | Higher | Significantly Lower |
| Oral Swab | QIAamp Viral RNA Mini Kit | Lower | Higher |
| Oral Swab | OxGEn RNA Kit | Lower | Higher |
A 2023 comparative study on RNA extraction from healthy individuals found that a modified manual acid guanidinium thiocyanate-phenol-chloroform (AGPC) method yielded a significantly higher amount of RNA from blood samples compared to commercial silica column-based kits (QIAamp and OxGEn). However, the purity of the AGPC extracts was significantly lower, which could render them unsuitable for sensitive downstream applications [25]. For oral swabs, the manual AGPC method also had lower purity compared to the commercial kits [25].
Speed, Throughput, and Practical Application
Table 2: Processing Time and Throughput of Automated Nucleic Acid Extractors [26]
| Extractor (Method) | Throughput (Samples/Run) | Preparation Time (16 samples) | Processing Time (16 samples) |
|---|---|---|---|
| Bioer GenePure Pro (Magnetic Beads) | 1-32 | ~25 min | ~35 min |
| Maxwell RSC 16 (Magnetic Beads) | 1-16 | ~35 min | ~42 min |
| KingFisher Apex (Magnetic Beads) | 1-96 | ~40 min | ~40 min |
| Manual Spin Column | Variable | N/A | ~100 min |
Magnetic bead-based systems offer substantial gains in speed and are easily automated. A 2024 evaluation of automated extractors showed processing times for 16 samples ranging from 35-42 minutes, significantly faster than manual column-based extraction, which took about 100 minutes [26]. Throughput varies by instrument, with some systems like the KingFisher Apex capable of processing 96 samples simultaneously [26]. Furthermore, a 2025 study reported a novel magnetic silica bead method (SHIFT-SP) that reduced the extraction time to just 6-7 minutes while achieving a similar or better yield than commercial bead and column-based methods [10].
Performance with Challenging and Degraded Samples
Table 3: DNA Extraction from Degraded Mammalian Museum Specimens [27]
| Extraction Method | Success Rate | Noted Advantages/Disadvantages |
|---|---|---|
| Phenol/Chloroform | Successful | Overall successful isolation. |
| QIAamp (Spin Column) | Successful | Outperformed magnetic bead isolations in these sample types. |
| Zymo MagBead (Magnetic Beads) | Lower Performance | Undigested tissue particles interfered with magnetic separation. |
A 2022 study on degraded mammalian museum specimens (53-130 years old) found that statistical analysis revealed the extraction method itself only explained 5% of the observed variation in outcomes, while specimen age explained 29% [27]. When isolation was successful, all methods produced quantifiable DNA. However, Qiagen spin columns and phenol-chloroform isolation outperformed Zymo magnetic bead isolations for these specific challenging samples, as particles from difficult-to-lyse materials interfered with the magnetic bead workflow [27].
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 4: Key Reagents and Their Functions in Nucleic Acid Extraction
| Reagent / Material | Primary Function |
|---|---|
| Chaotropic Salts (e.g., Guanidinium thiocyanate) | Denature proteins, inactivate nucleases, and facilitate binding of nucleic acids to silica [23] [10]. |
| Phenol-Chloroform | Organic solvent mixture used for liquid-liquid phase separation of nucleic acids from proteins and other cellular components [23] [24]. |
| Silica Membrane / Beads | Solid phase that binds nucleic acids in the presence of chaotropic salts and high ionic strength [23] [22] [24]. |
| Magnetic Beads | Silica-coated paramagnetic particles allowing for magnetic separation instead of centrifugation [23] [24]. |
| Proteinase K | Enzyme that digests and inactivates proteins and nucleases during the lysis step [27]. |
| Ethanol / Isopropanol | Used to precipitate nucleic acids from solution and in wash buffers to remove salts and other impurities [23] [28]. |
| Wash Buffer | Typically an ethanol-based solution used to remove contaminants while leaving nucleic acids bound to the silica matrix [23] [22]. |
| Elution Buffer | Low-salt aqueous solution (e.g., TE buffer, nuclease-free water) that disrupts the nucleic acid-silica interaction to release purified molecules [23] [22]. |
The historical progression from phenol-chloroform to silica columns and magnetic beads reflects the evolving needs of molecular biology. The choice of method is not a matter of identifying a single "best" technology, but rather of selecting the right tool for a specific application.
- Phenol-Chloroform remains valuable for its high yield and low cost, particularly when dealing with difficult samples or when budget is a primary constraint, provided that safety precautions are observed.
- Silica Spin Columns offer an excellent balance of speed, safety, purity, and convenience for routine, small-to-medium scale processing in most research laboratories.
- Magnetic Beads represent the forefront for high-throughput and automated applications, providing superior speed, scalability, and ease of integration into robotic liquid handling systems, which is critical for clinical diagnostics and large-scale genomic studies.
This comparison underscores the broader thesis that methodological advances in nucleic acid purification have consistently moved towards integrating greater simplicity, safety, and scalability without wholly abandoning the foundational principles of organic chemistry that made early genomics possible.
Protocols in Practice: A Detailed Guide to Organic and Solid-Phase Extraction Techniques
Nucleic acid extraction represents a critical first step in the NGS sample prep protocol and a wide array of molecular biology applications. Within this domain, organic extraction methods remain foundational techniques despite the proliferation of commercial solid-phase systems. This guide provides an objective comparison of two principal organic extraction protocols: the classic phenol-chloroform method and the acid guanidinium thiocyanate-phenol-chloroform (AGPC) approach. Framed within the broader thesis of organic versus solid-phase nucleic acid extraction research, we examine these methods through experimental data, protocol details, and practical considerations to inform researchers, scientists, and drug development professionals in their selection of appropriate extraction methodologies.
Methodological Foundations and Historical Context
The very first DNA isolation was performed in 1869 by Friedrich Miescher, who experimented with leucocytes from pus-infected bandages to isolate a novel molecule from cell nuclei [24]. Since this pioneering work, nucleic acid extraction techniques have evolved significantly. Organic extraction methods leverage the fundamental biochemical properties of nucleic acids, particularly their differential solubility in aqueous versus organic phases.
The phenol-chloroform method relies on a mixture of phenol, chloroform, and a small amount of isoamyl alcohol to separate DNA from proteins and lipids [24]. When added to a cell lysate, this mixture forms an emulsion that, upon centrifugation, separates into distinct phases with DNA partitioning into the upper aqueous layer due to its hydrophilic nature [24].
The acid guanidinium thiocyanate-phenol-chloroform (AGPC) extraction, originally devised by Chomczynski and Sacchi in 1987, represents a refinement specifically optimized for RNA isolation [29] [30]. This single-step method combines the denaturing power of guanidinium thiocyanate with the extraction efficiency of phenol-chloroform to rapidly isolate intact RNA while effectively inhibiting RNases [30].
Detailed Experimental Protocols
Phenol-Chloroform DNA Extraction Protocol
The phenol-chloroform DNA purification method effectively removes proteins and lipids from nucleic acids through differential solubility in immiscible solvents [31]. Below is the detailed experimental protocol:
- Sample Preparation: Homogenize the biological sample for complete cell lysis. For cells, resuspend in an appropriate volume of lysis buffer (e.g., TE buffer with SDS and proteinase K). For tissues, grind in liquid nitrogen before resuspending in lysis buffer [31].
- Cell Lysis: Incubate at 55°C for 1-2 hours or until the sample is completely lysed. Add proteinase K to the lysis buffer if necessary to facilitate protein digestion [31].
- Phenol-Chloroform Extraction: Add one volume of phenol:chloroform:isoamyl alcohol (25:24:1 ratio) to the lysed sample. Vortex or shake thoroughly for approximately 20 seconds to form an emulsion [31].
- Phase Separation: Centrifuge at room temperature for 5 minutes at 16,000 × g. This results in distinct layers: a lower organic phase containing denatured proteins and lipids, an interface, and an upper aqueous phase containing DNA. Carefully remove the upper aqueous phase without disturbing the interface or organic phase [31].
- Ethanol Precipitation:
- Add 1 μL glycogen (20 μg/μL), 0.5× volume of 7.5 M NH₄OAc (ammonium acetate), and 2.5× volume of 100% ethanol to the aqueous phase [31].
- Place the tube at -20°C overnight or at -80°C for at least 1 hour to precipitate DNA [31].
- Centrifuge at 4°C for 30 minutes at 16,000 × g to pellet the DNA.
- Carefully remove the supernatant without disturbing the pellet.
- Wash the pellet with 150 μL of 70% ethanol, centrifuge again, and carefully remove the supernatant.
- Air-dry the pellet for 5-10 minutes at room temperature or use a SpeedVac concentrator for 2 minutes [31].
- DNA Resuspension: Resuspend the purified DNA pellet in 50-100 μL of TE buffer or nuclease-free water by pipetting up and down 30-40 times [31].
- Quality Assessment: Measure DNA concentration and purity using spectrophotometry (e.g., Nanodrop) or by running an aliquot on an agarose gel [31].
Figure 1: Phenol-Chloroform DNA Extraction Workflow
Acid Guanidinium Thiocyanate-Phenol-Chloroform (AGPC) RNA Extraction Protocol
The AGPC method isolates RNA through phase separation under acidic conditions, which preferentially partitions RNA into the aqueous phase while DNA remains in the organic phase [29]. A modified manual AGPC protocol for blood and oral swab samples demonstrates the contemporary application of this method [25]:
- Sample Preparation and Lysis:
- For 200 μL of blood sample, add 925 μL of 1X RBC lysis buffer and incubate at room temperature for 10 minutes [25].
- Centrifuge at 1400 rpm for 10 minutes at 25°C. Discard the supernatant [25].
- Add 1000 μL of 1X RBC lysis buffer to the residue, incubate for 5 minutes at 25°C, then centrifuge at 3000 rpm for 2 minutes [25].
- Wash with 1000 μL of DPBS and centrifuge at 3000 rpm for 2 minutes. Discard the supernatant [25].
- Acid-Guadinidium Thiocyanate-Phenol Extraction:
- Add 1200 μL of homemade TRIzol reagent (containing water-saturated phenol, glycerol, sodium acetate, guanidine thiocyanate, and ammonium thiocyanate) to the residue to resuspend the cells [25].
- Add 200 μL of chloroform and vortex for 15 seconds [25].
- Centrifuge at 13,000 rpm for 10 minutes at 4°C. This results in phase separation with RNA in the upper aqueous phase [25].
- RNA Precipitation:
- RNA Wash:
- RNA Elution:
- Quality Assessment: Quantify RNA using spectrophotometry and confirm integrity by agarose gel electrophoresis [25].
Figure 2: AGPC RNA Extraction Workflow
Comparative Performance Analysis
Quantitative Comparison of Extraction Methods
The following tables summarize experimental data comparing the performance of organic extraction methods with solid-phase alternatives across different sample types and metrics.
Table 1: Performance comparison of DNA extraction methods from degraded human remains (n=25 samples) [32]
| Extraction Method | Small Human Target Quantity | Large Human Target Quantity | Degradation Index | Number of Reportable Loci | Overall Performance |
|---|---|---|---|---|---|
| Organic (Phenol-Chloroform) | Highest recovery | Superior recovery | Most favorable | Highest allele count | Best performing |
| Silica in Suspension | Good recovery | Good recovery | Favorable | Good allele count | Good performance |
| High Pure Silica Columns | Moderate recovery | Moderate recovery | Moderate | Moderate allele count | Moderate performance |
| InnoXtract Bone | Moderate recovery | Moderate recovery | Moderate | Moderate allele count | Moderate performance |
| PrepFiler BTA (Automated) | Moderate recovery | Moderate recovery | Moderate | Moderate allele count | Moderate performance |
Table 2: RNA extraction comparison between manual AGPC and commercial kits from blood and oral swabs (n=25 healthy individuals) [25]
| Extraction Method | Sample Type | Yield (ng/μL) | Purity (260/280 nm) | Statistical Significance |
|---|---|---|---|---|
| Manual AGPC | Blood | Highest | Lower | p < 0.0001 |
| Manual AGPC | Oral Swab | Highest | Lower | p < 0.0001 (vs QIAamp), p < 0.001 (vs OxGEn) |
| QIAamp Kit | Blood | Lower | Higher | p < 0.0001 |
| QIAamp Kit | Oral Swab | Lower | Higher | p < 0.0001 |
| OxGEn Kit | Blood | Lower | Higher | p < 0.0001 |
| OxGEn Kit | Oral Swab | Lower | Higher | p < 0.001 |
Table 3: Technical comparison of nucleic acid extraction methods [24] [11]
| Parameter | Phenol-Chloroform | AGPC | Silica Spin Columns | Magnetic Beads |
|---|---|---|---|---|
| Typical Yield | High | Very High | Moderate | Moderate to High |
| Purity | Good | Good for RNA | High | High |
| Processing Time | Long (several hours) | Moderate (4h to completion) | Short (25 min) | Very Short (6-7 min) |
| Cost Efficiency | High | High | Moderate | Moderate to High |
| Hazardous Materials | Yes (phenol, chloroform) | Yes (phenol, chloroform) | Minimal | Minimal |
| Automation Potential | Low | Low | Moderate | High |
| Throughput Capacity | Low | Low | High | Very High |
| Suitability for Small RNAs | Limited | Excellent | Poor (<200 nucleotides) | Good |
Key Experimental Findings
DNA Recovery from Challenging Samples: Organic extraction by phenol-chloroform/isoamyl alcohol demonstrated superior performance in forensic applications involving degraded skeletal remains, outperforming four other methods including silica-based approaches in both quantification metrics and DNA profile results [32].
RNA Yield Versus Purity Trade-off: The manual AGPC method showed significantly higher RNA yields from both blood and oral swab samples compared to commercial kits (p < 0.0001), but with correspondingly lower purity metrics [25]. This suggests a trade-off between quantity and quality that researchers must consider based on their downstream applications.
Efficiency in Modern Implementations: Recent innovations in magnetic silica bead-based extraction have achieved remarkable efficiency, with one method (SHIFT-SP) extracting nearly all nucleic acid from samples in just 6-7 minutes while maintaining compatibility with both DNA and RNA [10].
pH Optimization for Binding: Research on magnetic silica bead systems demonstrates that lower pH (4.1) significantly enhances nucleic acid binding efficiency, achieving 98.2% of input DNA bound within 10 minutes compared to 84.3% at pH 8.6 [10].
The Scientist's Toolkit: Essential Research Reagents
Table 4: Key reagents for organic nucleic acid extraction protocols
| Reagent | Function | Protocol Specificity |
|---|---|---|
| Phenol | Denatures and extracts proteins; carboxic acid that is flammable, corrosive, and toxic [24] | Common to both protocols |
| Chloroform | Extracts lipids; enhances phase separation [31] [29] | Common to both protocols |
| Isoamyl Alcohol | Reduces foaming during extraction [29] | Common to both protocols |
| Guanidinium Thiocyanate | Chaotropic agent that denatures proteins, inactivates RNases [29] | AGPC-specific |
| Sodium Acetate (pH 5.0) | Provides acidic conditions for RNA partitioning [29] | AGPC-specific |
| Proteinase K | Digests and inactivates cellular nucleases [31] | Phenol-chloroform specific |
| Glycogen | Carrier for nucleic acid precipitation [31] | Common to both protocols |
| Ammonium Acetate | Salt for ethanol precipitation [31] | Phenol-chloroform specific |
| Isopropanol | Precipitates nucleic acids [29] [25] | AGPC-specific |
| Ethanol | Precipitates and washes nucleic acids [31] | Common to both protocols |
Discussion: Organic vs. Solid-Phase Extraction in Contemporary Research
Advantages and Limitations in Practice
Organic extraction methods offer distinct advantages that maintain their relevance in modern laboratories:
- Cost-Effectiveness: Both phenol-chloroform and AGPC methods are relatively inexpensive compared to commercial kits, making them preferable for resource-limited settings [31] [25].
- Recovery Efficiency: Organic extraction consistently demonstrates superior recovery of nucleic acids from challenging, degraded samples as evidenced by forensic applications [32].
- RNA Integrity: The AGPC method provides higher purity RNA recovery and better preserves short RNA species (<200 nucleotides) such as siRNA, miRNA, and tRNA that may be lost in column-based systems [29].
However, these methods present significant limitations:
- Labor Intensity: Both protocols are manual, laborious processes with multiple steps requiring careful handling [31].
- Safety Concerns: Phenol and chloroform are hazardous, toxic materials requiring special handling and disposal considerations [11].
- Throughput Constraints: Organic methods are not amenable to high-throughput processing or automation, limiting their utility in large-scale studies [11].
Method Selection Guidelines
The choice between organic and solid-phase extraction methods depends on specific research requirements:
- For Maximum Yield and Cost-Effectiveness: Organic methods, particularly AGPC for RNA, deliver superior recovery at lower cost [25].
- For High-Throughput Applications: Magnetic bead-based systems offer rapid processing (as little as 6-7 minutes) and excellent automation compatibility [10].
- For Challenging or Degraded Samples: Phenol-chloroform extraction outperforms solid-phase methods for forensic and ancient DNA applications [32].
- For Short RNA Species: AGPC remains the method of choice for miRNA, siRNA, and tRNA studies where column-based systems fail [29].
- For Routine Applications: Silica spin columns provide a balance of convenience, purity, and adequate yield for standard molecular biology workflows [11].
Organic extraction methods, particularly phenol-chloroform and AGPC protocols, continue to hold significant value in molecular biology despite the proliferation of solid-phase alternatives. The experimental data presented demonstrates that organic methods consistently achieve higher nucleic acid yields, particularly from challenging or degraded samples, while remaining cost-effective. However, this advantage comes with trade-offs in throughput, safety, and purity that researchers must carefully evaluate based on their specific applications, sample types, and available resources. As nucleic acid extraction technologies evolve, the integration of organic chemistry principles with solid-phase convenience—exemplified by modern magnetic bead systems—promises to further blur these methodological boundaries, ultimately providing researchers with an expanding toolkit for nucleic acid purification across diverse research and diagnostic applications.
Solid-Phase Extraction (SPE) remains a cornerstone technique in analytical laboratories, serving as a critical sample preparation step across diverse fields including pharmaceuticals, environmental testing, food safety, and clinical diagnostics [33]. The fundamental principle of SPE involves selectively retaining target analytes on a solid sorbent while removing interfering matrix components, thereby purifying and concentrating samples for subsequent analysis. As analytical demands have evolved, so too have SPE technologies, with three primary formats emerging as dominant: silica spin columns, microplates, and magnetic bead technology. Within the specific context of nucleic acid extraction research, these SPE variants facilitate the isolation of DNA and RNA from complex biological matrices, enabling downstream applications from basic PCR to advanced next-generation sequencing [34] [35]. The choice between these methodologies significantly impacts not only workflow efficiency and cost, but also data quality and experimental outcomes, making a comprehensive comparative understanding essential for researchers, scientists, and drug development professionals.
Silica Spin Columns
Spin columns represent the traditional workhorse of nucleic acid extraction, operating on the principle of selective binding to a silica membrane under high-salt conditions [34]. The process involves sequential centrifugation steps: cellular lysis to release nucleic acids, binding to the silica membrane in the presence of chaotropic salts, washing to remove contaminants, and final elution in a low-salt buffer or water [11] [34]. Their simplicity and minimal equipment requirements (only a standard microcentrifuge) make them widely accessible [36]. However, their design presents inherent limitations for high-throughput applications, as they are inherently single-tube formats with limited automation compatibility [37].
Microplates
Microplate-based SPE systems represent a scalable adaptation of the spin column principle, utilizing 96-well plates containing silica or other functionalized sorbents [38]. Processing typically employs vacuum manifolds or centrifugation systems capable of handling all wells simultaneously, offering a significant throughput advantage over individual spin columns [11]. This format is particularly valuable in pharmaceutical and clinical settings where processing hundreds of samples is routine. While more efficient than single columns, microplates still face challenges related to potential membrane clogging and can require substantial manual liquid handling unless integrated with robotic systems [11].
Magnetic Bead Technology
Magnetic bead technology, a more recent innovation, utilizes paramagnetic particles coated with a silica or other functionalized surface that binds nucleic acids reversibly [11] [35]. The core mechanism, known as Solid Phase Reversible Immobilization (SPRI), occurs in the presence of polyethylene glycol (PEG) and salt [37]. The magnetic property of the beads enables separation using a magnetic field, eliminating the need for centrifugation or vacuum filtration [37] [35]. This fundamental difference facilitates seamless automation, as beads can be moved through wash steps while immobilized, making the technology ideal for high-throughput workflows and integration with liquid handling robots [37] [36]. A key advantage is the ease of scaling reactions by simply adjusting bead-to-sample ratios, which also allows for flexible size selection of nucleic acids [37].
Comparative Performance Analysis
Quantitative Performance Metrics
Direct comparisons between these technologies reveal significant differences in performance characteristics, influencing their suitability for specific applications.
Table 1: Comprehensive Performance Comparison of SPE Variants
| Performance Characteristic | Silica Spin Columns | Microplates | Magnetic Beads |
|---|---|---|---|
| Recovery Yield | 70-85% [37] | Similar to spin columns | 94-96% [37] |
| DNA Size Range | 100 bp – 10 kb [37] | Similar to spin columns | 100 bp – 50 kb [37] |
| Throughput Capacity | Low (manual, single-tube) [37] [36] | High (96-well batch processing) [11] | High (96/384-well & full automation) [37] [36] |
| Automation Compatibility | No [37] | Limited (vacuum/centrifuge systems) [11] | Yes (full robotic integration) [37] [36] |
| Hands-on Time | Manual, per sample [36] | Moderate (batch processing) | Minimal (no centrifugation) [36] |
| Size Selection Capability | No [37] | Limited | Yes (via adjustable bead ratio) [37] |
| Cost per Sample | ~$1.75 [37] | Moderate | ~$0.90 [37] |
Application-Specific Performance Data
Experimental data from direct comparisons further illuminates the performance differences. A 2025 clinical study comparing boiling (a simple lysis method) versus magnetic bead extraction for HPV genotyping detection demonstrated the superior performance of magnetic bead technology. The positive detection rate for HPV using the magnetic bead method was 20.66%, significantly higher than the 10.02% achieved with the boiling method (P < 0.001) in a paired study of 639 specimens [39]. Furthermore, the magnetic bead method exhibited superior resistance to inhibitors like hemoglobin, maintaining detection even at 60 g/L hemoglobin concentration, whereas the simple method failed at 30 g/L [39]. Although this cost 13.14% more, it increased the detection rate by 106.19%, demonstrating excellent cost-effectiveness for clinical diagnostics [39].
For nucleic acid purification, magnetic bead systems like the HighPrep PCR Cleanup Kit demonstrate superior recovery rates of 94-96% compared to 70-85% for spin columns, with the additional advantage of fragment size selection by modulating the bead-to-sample ratio [37].
Table 2: Fragment Size Selection in Magnetic Bead-Based Purification
| Bead-to-Sample Ratio | DNA Fragment Size Retained |
|---|---|
| 0.6x | >500 bp |
| 0.8x | >300 bp |
| 1.0x | >100 bp |
| 1.8x | >50 bp |
Source: Adapted from MagBio Genomics HighPrep protocol [37]
Detailed Experimental Protocols
Protocol: Nucleic Acid Extraction via Magnetic Bead Technology
The following protocol, adapted from the NAxtra magnetic nanoparticle procedure [35] and HighPrep methodologies [37], outlines a standardized approach for automated nucleic acid extraction suitable for high-throughput applications.
Reagents and Equipment:
- Lysis buffer (e.g., customized buffer with RNase inhibitors) [35]
- Magnetic beads (e.g., silica-coated magnetic nanoparticles) [35]
- Wash buffers (typically ethanol-based) [37]
- Elution buffer (nuclease-free water or TE buffer) [37]
- KingFisher Flex Purification System or equivalent magnetic particle processor [35]
- 96-well deep-well plates
Procedure:
- Cell Lysis: Transfer 300 µL of sample to a 96-well deep-well plate. Add lysis buffer and mix thoroughly to release nucleic acids. Incubate for 5-10 minutes at room temperature [35].
- Binding: Add 1.8x volume of magnetic beads to the lysate. Mix thoroughly by pipetting or plate shaking. Incubate for 5 minutes at room temperature to allow nucleic acid binding [37].
- Separation: Transfer the plate to the magnetic processor. Engage the magnetic field for 2-5 minutes until beads form a pellet and the supernatant clears [37].
- Washing: Remove and discard the supernatant while maintaining the magnetic field. Add 200 µL of 80% ethanol wash buffer. Incubate for 30 seconds, then remove the wash solution. Repeat this wash step twice [37] [35].
- Drying: Air-dry the bead pellet for 3-5 minutes at room temperature to ensure complete ethanol evaporation. Avoid over-drying, which can reduce elution efficiency [37].
- Elution: Remove the plate from the magnetic field. Add 20-50 µL of nuclease-free water or elution buffer. Mix thoroughly and incubate for 2-5 minutes to resuspend beads and release nucleic acids [37].
- Final Separation: Return the plate to the magnetic field for 2 minutes. Transfer the clarified eluate containing purified nucleic acids to a clean collection plate [35].
Typical Duration: 12-18 minutes for 96 samples when automated [35].
Protocol: Solid-Phase Extraction for Pharmaceutical Compounds
This protocol, adapted from wastewater pharmaceutical analysis research [40], demonstrates the application of cartridge-based SPE for small molecule extraction, highlighting its relevance in environmental and pharmaceutical analysis.
Reagents and Equipment:
- Oasis HLB cartridges (60 mg/3 mL) or equivalent [40]
- Vacuum manifold
- Methanol (HPLC grade)
- Acidified water (pH 2 with HCl) [40]
- Nitrogen evaporator
Procedure:
- Conditioning: Pre-condition the HLB cartridge with 5 mL of methanol, followed by 5 mL of acidified water (pH 2) at a flow rate of 1 mL/min [40].
- Sample Loading: Load 100 mL of sample (adjusted to pH 2) onto the cartridge under vacuum [40].
- Washing: Rinse the cartridge with 5 mL of 10% methanol and 5 mL of ultra-pure water to remove interfering compounds [40].
- Elution: Elute the adsorbed analytes with 4-6 mL of 100% methanol into a collection tube [40].
- Concentration: Evaporate the eluate to dryness under a gentle nitrogen stream at 50°C. Reconstitute the residue in 1 mL of methanol [40].
- Filtration: Filter the reconstituted sample through a 0.22 µm nylon syringe filter prior to HPLC analysis [40].
The Scientist's Toolkit: Essential Research Reagent Solutions
Table 3: Key Reagents and Materials for Solid-Phase Extraction Workflows
| Item | Function | Example Applications |
|---|---|---|
| Hydrophilic-Lipophilic Balance (HLB) Cartridges | Retains both polar and non-polar compounds [40] | Pharmaceutical contaminant extraction from wastewater [40] |
| Silica-Coated Magnetic Beads | Paramagnetic particles with silica surface for nucleic acid binding [35] | High-throughput DNA/RNA extraction [37] [35] |
| Chaotropic Salts | Denature proteins and promote nucleic acid binding to silica [11] | Spin column nucleic acid purification [11] [34] |
| ENV+ and PHE SPE Sorbents | Specialized sorbents for multiplexed extraction of diverse analytes [41] | Untargeted adductomics from urine samples [41] |
| PCR Cleanup Magnetic Beads | Size-selective binding of nucleic acids via SPRI technology [37] | Post-amplification purification and size selection [37] |
Selection Guidelines and Future Perspectives
Technology Selection Framework
Choosing the appropriate SPE technology requires careful consideration of application requirements and laboratory constraints:
- Choose Silica Spin Columns when: Processing small sample numbers (1-24), working with limited budget, operating in minimally equipped laboratories, or when method development time is limited [34] [36].
- Choose Microplate Formats when: Processing medium to high sample volumes (96-384), utilizing vacuum manifold systems, and when consistent batch processing is prioritized [38].
- Choose Magnetic Bead Technology when: Throughput and automation are critical, working with challenging samples (low concentration/inhibitors), requiring high recovery yields, needing size selection capability, or when minimizing hands-on time is a priority [37] [36] [35].
Emerging Trends and Future Outlook
The SPE landscape continues to evolve, with several key trends shaping its trajectory. By 2025, vendors are prioritizing automation, miniaturization, and environmentally sustainable solutions [33]. The integration of SPE with other analytical techniques, particularly online SPE-LC-MS systems, is creating streamlined workflows that enhance analytical efficiency and reduce manual intervention [38]. The development of novel sorbent materials with improved selectivity continues to expand application possibilities, while the push toward miniaturization through microfluidic devices reduces solvent consumption and sample volume requirements [38]. These advancements, coupled with growing demand in pharmaceutical and environmental sectors, project a market value exceeding $900 million by 2033, with magnetic bead technology and automated systems expected to capture increasing market share [38].
In molecular research, the purity and integrity of isolated nucleic acids are foundational to the success of downstream applications, from routine PCR to cutting-edge next-generation sequencing. The choice of extraction method is a critical decision point that balances yield, purity, scalability, and safety. This guide objectively compares the two predominant philosophies in nucleic acid isolation: organic extraction and solid-phase extraction, with a focus on tailoring these methods to specific sample types including blood, stool, tissues, and cultured cells. Organic extraction, historically the gold standard, relies on liquid-phase separation using phenol-chloroform, while solid-phase methods utilize a solid substrate, most commonly silica, to bind and purify nucleic acids [11]. The following sections provide a detailed comparison based on experimental data, outline sample-specific optimized protocols, and present key reagent solutions to equip researchers with the tools for informed methodological selection.
Method Comparison: Organic vs. Solid-Phase Extraction
The performance of any extraction method is quantified through metrics such as nucleic acid yield, purity, processing time, and suitability for automation. The table below summarizes the core characteristics of the primary extraction techniques.
Table 1: Comparative Analysis of Nucleic Acid Extraction Methods
| Feature | Organic Extraction | Spin Column (Solid-Phase) | Magnetic Particle (Solid-Phase) |
|---|---|---|---|
| Core Principle | Liquid-liquid phase separation using phenol-chloroform [11] | Silica membrane in a column binds nucleic acids under chaotropic salts [11] | Silica-coated paramagnetic beads bind nucleic acids [11] |
| Typical Yield & Purity | High yield, well-established for difficult samples [11] | High purity, but yield can be low with incomplete lysis or clogging [11] | High purity, efficient for a range of sample types [11] [26] |
| Throughput | Low, not amenable to automation [11] | High, amenable to 96-well plates and automation [11] | Very high, most easily automated (e.g., KingFisher Apex) [11] [26] |
| Processing Time | Laborious and time-consuming due to manual handling [11] | Rapid and straightforward procedure [11] | Rapid collection and resuspension with magnets [11] |
| Key Advantages | Gold standard; well-established protocols; effective protein denaturation [11] | Convenient, ready-to-use kit format; flexible for centrifugation or vacuum [11] | Amenable to full automation; no filter clogging; no organic hazardous waste [11] |
| Key Disadvantages | Use of hazardous chemicals; difficult to automate; labor-intensive [11] | Membrane can clog; sample input limitations; automation equipment can be expensive [11] | Viscous samples can impede beads; can be laborious manually; risk of bead carryover [11] |
Experimental data from a 2024 study comparing automated nucleic acid extractors provides quantitative support for these comparisons. The study evaluated systems like the KingFisher Apex (magnetic particles) and Maxwell RSC (magnetic particles in pre-packed cartridges) against manual column-based extraction. It highlighted that instruments utilizing magnetic particle-based solid-phase extraction demonstrated high efficiency and reduced inter-sample variability, which is crucial for high-throughput research [26]. Furthermore, the integration of an additional mechanical lysis step, such as bead-beating, was found to be critical for efficient extraction from complex matrices like stool, leading to a greater representation of Gram-positive bacteria in subsequent microbiome analyses [26].
Sample-Specific Experimental Protocols
The optimal extraction of nucleic acids requires precise, sample-specific preparation and lysis techniques to maximize yield and quality.
Cultured Cells
- Cell Preparation: For adherent cell lines, detach cells using a method appropriate for your downstream application (e.g., trypsin, Accutase, or a cell scraper). For cells in suspension, proceed directly. Transfer cells to a conical tube, dissociate any clumps by pipetting, and perform a cell count and viability analysis [42].
- Nucleic Acid Extraction: Pellet cells by centrifugation (300-400 x g for 4-5 minutes). Resuspend the pellet in the appropriate lysis buffer. For organic extraction, homogenize in a phenol-containing solution. For solid-phase extraction, lyse in a buffered solution containing RNase inhibitors and a high concentration of chaotropic salt, then bind to the silica membrane or beads according to the kit protocol [11] [42].
Stool Samples
- Sample Homogenization: Stool samples are complex and require rigorous homogenization. For robust lysis of diverse microbial communities, mechanical disruption with bead-beating is the gold standard. One protocol involves adding a 300 µL aliquot of preserved stool to a tube containing lysing matrix E and using a homogenizer like the FastPrep-24 at 6.0 m/s for 40 seconds [26].
- Inhibition Management: Stool contains PCR inhibitors that must be removed. Both organic extraction and specialized solid-phase kits (e.g., Maxwell RSC Fecal Microbiome DNA Kit, MagMAX Microbiome Ultra Kit) are effective at removing these inhibitors. The magnetic particle method is particularly advantageous as it avoids filter clogging [11] [26].
Lymphoid and Non-Lymphoid Tissues
- Creating a Single-Cell Suspension:
- Lymphoid Tissue (Spleen, Thymus, Lymph Nodes): Harvest tissue into a dish containing buffer. Mechanically disrupt the tissue by pressing with the plunger of a syringe or mashing between two frosted glass slides. Pass the resulting suspension through a nylon mesh cell strainer to eliminate clumps and debris [42].
- Non-Lymphoid Tissue: Harvest and mince the tissue into 2-4 mm pieces using scissors or a scalpel. Digest using appropriate enzymes (e.g., collagenase) diluted in PBS, incubating at the optimal temperature and time. After digestion, disperse cells by gentle pipetting and filter through a cell strainer [42].
- Nucleic Acid Extraction: Pellet the cells from the single-cell suspension by centrifugation. The resulting pellet can be processed using organic or solid-phase methods. For fibrous tissues, organic extraction may be more effective at breaking down the complex structure, though many solid-phase kits are also validated for tissue use [11] [42].
Blood Samples
- Peripheral Blood Mononuclear Cell (PBMC) Isolation: Dilute whole blood at least 1:1 with PBS. Carefully underlay the diluted sample with an equal volume of a density separation medium like Ficoll-Paque. Centrifuge at 400 x g for 20 minutes at room temperature with the brake OFF. Harvest the mononuclear cell layer at the interface into a new tube. Wash cells with PBS and centrifuge at 300-400 x g for 4-5 minutes [42] [43].
- Whole Blood Lysis Method: As a faster alternative to density separation, erythrocytes can be lysed using an osmotic shock method. Resuspend the pelleted cell pack from centrifuged blood in a commercial ammonium chloride-based lysis buffer (e.g., ACK, PharmLyse) at a 1:10 ratio. Gently rock for 10 minutes at room temperature. Pellet the remaining leukocytes by centrifugation and wash with PBS [43].
- Nucleic Acid Extraction: The resulting PBMC or leukocyte pellet is ready for nucleic acid extraction. Solid-phase extraction kits designed for blood are highly effective and avoid the use of hazardous solvents [11].
The Scientist's Toolkit: Key Research Reagent Solutions
Successful experimentation relies on a suite of reliable reagents and materials. The following table details essential solutions for sample preparation and nucleic acid extraction.
Table 2: Essential Reagents and Kits for Sample Processing and Extraction
| Reagent / Kit | Primary Function | Sample Application |
|---|---|---|
| Accutase / Trypsin-EDTA [42] | Enzymatic detachment of adherent cells from culture surfaces. | Cultured Cells |
| Ficoll-Paque [42] [43] | Density gradient medium for isolation of peripheral blood mononuclear cells (PBMCs). | Blood |
| Ammonium Chloride Lysis Buffer (ACK) [43] | Osmotic lysis of red blood cells to enrich for white blood cells. | Blood |
| Collagenase / Other Proteases [42] | Enzymatic digestion of extracellular matrix to dissociate tissue into single cells. | Tissues |
| DNA/RNA Shield Preservative [26] | Immediate stabilization and protection of nucleic acids in samples, preventing degradation. | Stool, Tissues |
| Phenol-Chloroform Solutions [11] | Organic denaturation and removal of proteins from a cell lysate. | All Types |
| Silica-Membrane Spin Columns (e.g., in various kits) [11] [26] | Bind nucleic acids in the presence of chaotropic salts; impurities are washed away. | All Types |
| Silica-Coated Magnetic Beads (e.g., MagMAX kits) [11] [26] | Paramagnetic beads bind nucleic acids for automated purification in magnetic fields. | All Types |
| Proteinase K [26] | Broad-spectrum serine protease that degrades proteins and inactivates nucleases. | Stool, Tissues |
The choice between organic and solid-phase extraction methods is not a one-size-fits-all decision but a strategic one, heavily influenced by sample type, throughput requirements, and safety considerations. While organic extraction remains a powerful, gold-standard method for challenging samples due to its effective protein denaturation, solid-phase methods, particularly magnetic particle-based technologies, dominate in high-throughput and automated environments due to their speed, convenience, and reduced use of hazardous chemicals [11] [26]. As demonstrated by the sample-specific protocols, the initial processing of blood, stool, tissues, or cultured cells is equally critical to the final extraction step. By understanding the principles, trade-offs, and optimized workflows outlined in this guide, researchers and drug development professionals can make informed decisions to ensure the consistent isolation of high-quality nucleic acids, thereby solidifying the foundation for reliable and impactful scientific results.
The integration of automation into nucleic acid extraction represents a pivotal advancement in life sciences, particularly for drug discovery and clinical diagnostics where throughput, reproducibility, and speed are paramount. The selection of extraction methodology—organic versus solid-phase—directly influences downstream analytical success, experimental throughput, and ultimately, research outcomes. Isolating high-quality nucleic acids is the most critical step for successfully performing a broad range of assays, from RT-qPCR and microarray analysis to next-generation sequencing techniques [11]. As laboratories evolve toward automated visions with self-enclosed robotic hoods replacing traditional benches, understanding the performance characteristics of different extraction methods within automated workflows becomes essential for researchers, scientists, and drug development professionals [44].
This comparison guide objectively evaluates organic and solid-phase extraction methodologies within the context of automated, high-throughput processing environments. We examine experimental data comparing DNA yield, purity, and downstream analytical performance across systems, providing detailed methodologies and quantitative comparisons to inform laboratory selection and workflow integration. The transition toward automation addresses significant challenges in manual processing, including human error, sample-to-sample variation, and limited throughput, which directly affect research reproducibility [26]. With automated systems capable of processing hundreds of samples simultaneously with minimal manual intervention, researchers can allocate valuable time to more complex analytical tasks while maintaining consistency across experimental batches [45].
Nucleic Acid Extraction Methodologies: Core Principles and Mechanisms
Organic Extraction: The Established Benchmark
Organic extraction of nucleic acids represents the historical gold standard for RNA and DNA isolation, utilizing phase separation to partition nucleic acids from cellular proteins. This technique requires homogenization of biological samples in a phenol-containing solution, typically phenol-chloroform. The mixture is immiscible with water, forming two distinct phases when centrifuged: a lower organic phase and phase interface containing denatured proteins, and an upper aqueous phase containing nucleic acids [11]. The partitioning of DNA and RNA is pH-dependent; at pH greater than 7.0, both RNA and DNA resolve in the aqueous phase, while at pH less than 7.0, DNA denatures and precipitates into the organic phase with RNA remaining aqueous [11]. The aqueous phase is carefully removed by pipetting to avoid interface contamination, followed by alcohol precipitation and rehydration for further analysis.
The advantages of organic extraction include its well-established, straightforward protocols that rapidly denature proteins and stabilize RNA, making it applicable to diverse sample types from human tissues to cell cultures [11]. However, significant limitations emerge in high-throughput contexts: the method is not readily amenable to automation, involves laborious manual processing, and requires careful management of hazardous chemical waste [11]. Despite these limitations, organic extraction remains a performance benchmark, with recent forensic studies on degraded human remains demonstrating its superior performance in both DNA quantification and profile quality compared to silica-based methods [32].
Solid-Phase Extraction: Modern Approaches for Automation
Solid-phase extraction techniques have revolutionized nucleic acid isolation through binding mechanisms that interface efficiently with automated platforms. These methods encompass several technological approaches:
Spin Column Extraction utilizes filter-based columns with silica or glass fiber membranes to bind nucleic acids. Samples are lysed in buffered solution containing RNase inhibitors and chaotropic salts, with lysates passed through silica membranes via centrifugation. RNA binds to silica at appropriate pH, impurities are washed away, and pure RNA is eluted with RNase-free water [11]. This approach offers procedural simplicity and convenience in kit format, with flexibility for centrifugation or vacuum processing. Limitations include membrane clogging with excessive sample, potential genomic DNA contamination, and low yields from incomplete lysis [11].
Magnetic Particle Extraction employs paramagnetic beads with silica coatings for nucleic acid binding. Cells are lysed with RNase inhibitors, incubated with magnetic beads for RNA binding, then placed near a magnetic field for collection. After supernatant removal, beads undergo washing and resuspension before RNA elution [11]. This strategy is particularly amenable to automation and high-throughput processing, with rapid magnetic collection steps that reduce clogging concerns and eliminate organic solvents. Challenges include impeded bead migration in viscous samples and potential RNA contamination from residual beads [11].
Table 1: Comparison of Fundamental Nucleic Acid Extraction Methodologies
| Parameter | Organic Extraction | Spin Column Extraction | Magnetic Particle Extraction |
|---|---|---|---|
| Core Principle | Liquid-liquid phase separation | Solid-phase adsorption to silica membrane | Solid-phase adsorption to magnetic silica particles |
| Throughput Capacity | Low, not amenable to automation | Moderate to high, amenable to automation | High, highly amenable to automation |
| Processing Time | Lengthy due to manual steps | Moderate, protocol-dependent | Fastest, especially in automated systems |
| Hazardous Waste | Yes, requires special management | Minimal | None |
| Risk of Clogging | Not applicable | Yes, with large or incomplete homogenized samples | No |
| Sample Types | Diverse, including tough tissues | Limited by lysis efficiency and membrane capacity | Diverse, though viscous samples problematic |
| Downstream Application Flexibility | Broad compatibility | Broad compatibility | Broad compatibility |
Comparative Experimental Data: Performance Across Automated Platforms
DNA Yield and Purity in Microbiome Studies
Recent comparative studies evaluating automated nucleic acid extraction systems provide critical performance data for platform selection. A 2024 comprehensive assessment of three commercial automated extractors—Bioer GenePure Pro, Promega Maxwell RSC 16, and ThermoFisher KingFisher Apex—evaluated their performance using both human fecal samples and mock microbial communities, with bead-beating mechanical lysis compared to lysis buffer alone [26]. The research demonstrated that all three commercial extractors exhibited differences in yield, inter-sample variability, and subsequent sequencing readouts, significant considerations for researchers undertaking human fecal microbiota research [26].
Differential abundance analysis and comparison of prevalent bacterial species revealed a greater representation of Gram-positive bacteria in samples subjected to mechanical lysis, regardless of sample type, highlighting the critical importance of lysis efficiency in extraction completeness [26]. Bead-beating provided incremental yield improvement for effectively lysing and extracting DNA from stool samples compared to lysis buffer alone, confirming mechanical lysis as the gold standard for effectively disrupting diverse microbial cell types, including spores [26].
Table 2: Performance Metrics of Automated Nucleic Acid Extraction Systems
| Extraction System | Technology Base | Throughput (samples/run) | Processing Time (16 samples) | Bead-Beating Compatibility | Total Raw Reads (With Bead-Beating) | Elution Volume |
|---|---|---|---|---|---|---|
| Bioer GenePure Pro | Magnetic bead-based | 1-32 | ~35 minutes | Yes/No | 1,482,643 | 50 µL |
| Promega Maxwell RSC 16 | Magnetic bead-based | 1-16 | ~42 minutes | Yes/No | 1,753,841 | 50-100 µL |
| ThermoFisher KingFisher Apex | Magnetic bead-based | 1-96 | ~40 minutes | Yes | 1,223,111 | 50-200 µL |
| Manual Column Extraction | Spin column | Variable | ~100 minutes | Yes | 1,274,852 | 50-100 µL |
Forensic Applications: Degraded Sample Performance
Forensic genetics laboratories face particular challenges with degraded human remains, requiring efficient high-throughput methods that recover often-fragmented DNA. A comprehensive comparison of five DNA extraction protocols on 25 different degraded skeletal remains evaluated organic extraction, silica in suspension, High Pure Nucleic Acid Large Volume silica columns (Roche), InnoXtract Bone (InnoGenomics), and PrepFiler BTA with AutoMate Express robot (ThermoFisher) [32]. Researchers analyzed five DNA quantification parameters and five DNA profile parameters, with results indicating that organic extraction by phenol/chloroform/isoamyl alcohol performed best in both quantification and DNA profile results [32]. However, Roche silica columns were identified as the most efficient method, balancing performance with practical implementation considerations [32].
Workflow Integration: From Manual Processing to Automated Systems
Transitioning to Automated Workflows
The integration of extraction methodologies into automated workflows transforms laboratory efficiency and data quality. Automated nucleic acid extractors are marketed to reduce manual handling and, since their introduction in the early 2000s, have been implemented in forensic science and clinical studies for viral and bacterial pathogens [26]. These benchtop systems primarily function as liquid handlers that aliquot reagents and samples, perform nucleic acid purification using DNA-binding magnetic beads, and incorporate limited heating capacity to optimize enzymatic activities such as proteinase K digestion [26].
Most automated extractors traditionally lacked integrated bead-beating capability, potentially compromising microbiome results where mechanical lysis is regarded as essential for effective disruption of diverse microbial cell types [26]. However, newer systems increasingly incorporate this functionality, as evidenced by the KingFisher Apex's requirement for bead-beating and the optional compatibility of the GenePure Pro and Maxwell RSC systems [26]. This evolution addresses a critical methodological requirement for comprehensive microbiota analysis.
The Software Backbone: Data Management in Automated Extraction
The automation infrastructure extends beyond robotic arms to encompass sophisticated software systems that track samples, analyze results, and capture data at each process step. This "ghost in the machine" often automatically makes decisions to discard, repeat, or advance samples to subsequent workflow stages, alerting human scientists only when ambiguous results require input [44]. The scale of data generation in automated workflows is staggering—a single automated molecular cloning batch with 1,152 samples can create over 1.1 million unique metadata and data points [44]. Without robust software tracking all samples and associated metadata from DNA in transfection plates through protein production, managing these complex workflows would be virtually impossible [44].
Well-designed automation software understands each process step, the type of data captured, and recognizes when operations deviate from planning. Systems perform automatic long-term monitoring to assess assay performance across plates and weeks, detecting problems before human observation would identify them [44]. This software infrastructure becomes particularly vital for maintaining traceability and audit reporting, automatically tracking every process step and uploading analytical results to corresponding samples [44].
Experimental Protocols: Methodologies for Automated Extraction
Automated DNA Extraction from Fecal Samples with Bead-Beating
The following detailed protocol was utilized in the comparative study of automated extractors [26], providing a robust methodology for microbial DNA extraction:
Sample Preparation:
- Fresh fecal samples were collected and processed within 2 hours of collection.
- In an anaerobic chamber (91% N₂, 5% CO₂, 4% H₂), 1 gram (w/w) of stool was aliquoted into DNA/RNA Shield Fecal Collection Tubes containing 9 mL of preservation reagent.
- Samples were vortexed and stored at -80°C until DNA extraction.
Homogenization and Lysis:
- Frozen fecal samples were thawed at room temperature.
- 300 µL of fecal DNA shield mixture was aliquoted per replicate.
- Samples underwent mechanical lysis using the FastPrep-24 5G Bead Beating Grinder and lysis system at 6.0 m/s for 40 seconds.
Automated Extraction:
- Processed samples were loaded onto the automated extraction systems (GenePure Pro, Maxwell RSC 16, or KingFisher Apex) according to manufacturer specifications.
- Manufacturer-specific kits were utilized: MagaBio Fecal Pathogens DNA Purification Kit (Bioer), Maxwell RSC Fecal Microbiome DNA Kit (Promega), or MagMAX Microbiome Ultra Kit (ThermoFisher).
- Extraction protocols were followed without deviation unless otherwise specified.
- All centrifugations were performed at 14,000 × g unless specified.
DNA Elution and Storage:
- Extracted DNA was eluted in manufacturer-specified volumes (50-200 µL depending on system).
- DNA concentration and quality were measured using Qubit 4 fluorometer with dsDNA HS assay kit and NanoDrop One spectrophotometer.
- All extracted DNA was stored at -80°C before library preparation for 16S rRNA amplicon sequencing.
16S rRNA Amplicon Sequencing and Analysis
Library Preparation:
- Purified DNA underwent library preparation using Nextera DNA Library Prep Kit.
- 16S amplicon sequencing was performed on Illumina MiSeq with 2 × 300 bp paired-end sequencing.
- Primers 338F-5' (CCTACGGRRBGCASCAGKVRVGAAT) and 806R-5' (GGACTACNVGGGTWTCTAATCC) amplified the V3-V4 region of the 16S rRNA gene.
- PCR conditions included initial denaturation at 95°C for 3 minutes, followed by 28 cycles of 95°C for 30 seconds, 55°C for 30 seconds, and 72°C for 30 seconds, with final elongation at 72°C for 5 minutes.
Data Analysis:
- All sequenced samples, including kit controls, were analyzed to evaluate extraction system effects on fecal microbiota.
- For mock community samples, a total of 1,700,732 denoised sequences were analyzed.
- Differential abundance analysis compared prevalent bacterial species across extraction methods.
Visualization of Automated Workflows
The following workflow diagrams illustrate the logical relationships and process flows in automated nucleic acid extraction systems, highlighting key decision points and technological integrations.
Automated Nucleic Acid Extraction Workflow
The Scientist's Toolkit: Essential Research Reagent Solutions
Successful integration of extraction methodologies into automated workflows requires specific reagent systems and materials optimized for high-throughput processing. The following table details essential solutions and their functions within automated nucleic acid extraction protocols.
Table 3: Essential Research Reagent Solutions for Automated Nucleic Acid Extraction
| Reagent/Material | Function | Application Notes |
|---|---|---|
| DNA/RNA Shield Preservation Reagent | Stabilizes nucleic acids immediately after sample collection, preventing degradation | Critical for maintaining sample integrity between collection and processing; enables batch processing [26] |
| Lysis Buffer with Chaotropic Salts | Disrupts cell membranes, releases nucleic acids, and inactivates nucleases | Composition varies by sample type; often includes guanidine thiocyanate or hydrochloride [11] |
| Magnetic Silica Beads | Paramagnetic particles with silica coating for nucleic acid binding | Core component of magnetic particle systems; enable automated magnetic separation [11] [26] |
| Silica Membrane Columns | Solid-phase matrix for nucleic acid binding in spin column systems | Standard in many kit-based approaches; compatible with centrifugation or vacuum processing [11] |
| Proteinase K | Broad-spectrum serine protease for digesting contaminating proteins | Particularly important for tough samples (tissues, spores); often included in lysis step [26] |
| RNase Inhibitors | Protects RNA integrity during extraction process | Essential for RNA isolation; included in lysis buffers [11] |
| Wash Buffers (Ethanol-Based) | Removes contaminants, salts, and inhibitors while retaining bound nucleic acids | Typically contain ethanol or isopropanol; critical for purity of final eluate [11] [46] |
| Elution Buffers (Low Salt/TE) | Releases purified nucleic acids from binding matrices | Low ionic strength solutions (TE buffer or nuclease-free water); volume affects final concentration [11] |
The integration of nucleic acid extraction into automated workflow pipelines requires careful consideration of methodological strengths and limitations relative to application requirements. Organic extraction remains the performance benchmark for challenging samples, including degraded human remains, delivering superior DNA quantification and profile quality [32]. However, solid-phase extraction methods, particularly magnetic particle-based approaches, offer significant advantages in automated, high-throughput environments where processing speed, sample volume, and reduction of hazardous waste are prioritized [11] [26].
The comparative experimental data presented demonstrates that automated extraction systems differ meaningfully in DNA yield, inter-sample variability, and subsequent sequencing results, with mechanical lysis integration proving critical for comprehensive microbial representation [26]. Researchers must consider these performance characteristics alongside throughput requirements, sample types, and downstream applications when selecting extraction methodologies for automated workflows. As automation technologies evolve with improved integration of mechanical lysis and sophisticated software management, the throughput and reproducibility advantages of solid-phase extraction systems continue to expand, positioning them as the foundation for next-generation high-throughput bioscience research [44] [45].
Troubleshooting and Optimization: Maximizing Yield, Purity, and Efficiency in the Lab
Nucleic acid (NA) extraction is a foundational step in molecular diagnostics, PCR, microarray analysis, and next-generation sequencing workflows. The quality and yield of extracted DNA and RNA directly influence the sensitivity, accuracy, and reliability of all downstream analytical processes. Within research and drug development, the choice between organic and solid-phase extraction methods is pivotal, each presenting a unique profile of advantages and challenges. Solid-phase extraction, particularly silica-based methods, has gained widespread adoption due to its robustness and automatable nature. However, these methods are frequently plagued by pitfalls such as co-purification of PCR inhibitors, low nucleic acid yield, and degradation of the target molecule. These issues are exacerbated when processing complex samples, such as stool, processed food, or enriched whole blood, which contain inherent substances that interfere with extraction and amplification. This guide objectively compares the performance of various NA extraction techniques, with a focus on magnetic silica bead-based methods, to provide researchers and scientists with data-driven solutions to these common challenges. By framing this discussion within the broader thesis of organic versus solid-phase extraction research, we aim to deliver a critical evaluation of modern protocols that enhance efficiency, purity, and yield for advanced diagnostic and drug development applications.
Comparative Analysis of Nucleic Acid Extraction Methods
The performance of any molecular diagnostic assay is profoundly influenced by the upstream NA extraction process. This section provides a comparative evaluation of different extraction methodologies, highlighting their performance in terms of yield, purity, and operational efficiency.
Performance Metrics and Experimental Data
Table 1: Quantitative comparison of DNA extraction methods from recent studies.
| Extraction Method | Sample Type | Extraction Time | DNA Yield | Key Performance Findings | Source |
|---|---|---|---|---|---|
| SHIFT-SP (Magnetic Silica Bead) | Lysed sample (model DNA) | 6–7 min | ~98.2% binding efficiency | High yield, automation compatible, efficient for both DNA and RNA. | [10] |
| Commercial Bead-Based | Lysed sample (model DNA) | ~40 min | Similar DNA yield to SHIFT-SP | Similar yield but significantly longer processing time. | [10] |
| Commercial Column-Based | Lysed sample (model DNA) | ~25 min | Half the DNA yield of SHIFT-SP | Faster than other commercial methods but with substantially lower yield. | [10] |
| Combination Method | Chestnut rose juice/beverage | Not Specified | Highest performance | Best for processed foods; high quality but time-consuming and costly. | [14] |
| Non-commercial CTAB | Chestnut rose juice/beverage | Not Specified | High concentration, poor quality | Spectrophotometry and qPCR showed poor DNA quality. | [14] |
| Spin-Column (SC) | Spiked broiler feces | Not Specified | Higher purity/quality | Superior performance in LAMP and PCR assays. | [47] |
| Hotshot (HS) | Spiked broiler feces | Not Specified | Practical lower sensitivity | Most practical for resource-limited settings; lower sensitivity. | [47] |
Table 2: Comparison of three automated nucleic acid extraction systems for human stool samples.
| Extraction System | Bead-Beating Step | Key Microbiome Findings | Sample Type |
|---|---|---|---|
| Bioer GenePure Pro | With and without | Bead-beating increased yield and improved representation of Gram-positive bacteria. | Human fecal samples, Mock community |
| Maxwell RSC 16 | With and without | Bead-beating increased yield and improved representation of Gram-positive bacteria. | Human fecal samples, Mock community |
| KingFisher Apex | With and without | Bead-beating increased yield and improved representation of Gram-positive bacteria. | Human fecal samples, Mock community |
| Manual (Column-based) | Yes (for comparison) | Considered a standard for effective lysis of diverse microbial cell types. | Human fecal samples, Mock community |
Critical Insights from Comparative Data
The data from these independent studies reveal several critical trends. The SHIFT-SP method demonstrates that optimization of binding conditions, such as pH and mixing mode, can drastically reduce extraction time to under 10 minutes while achieving near-complete nucleic acid recovery [10]. This is a significant advancement over traditional commercial bead-based and column-based methods, which face a trade-off between time and yield.
For complex samples like stool, the inclusion of a mechanical lysis step (bead-beating) is crucial for the effective rupture of diverse microbial cell walls, including Gram-positive bacteria. Without this step, automated extractors yield a biased representation of the microbiome, regardless of the platform used [9]. In the context of processed foods, the Combination Method proved most effective but highlighted a universal challenge: the more complex the sample matrix and processing method, the greater the compromise between DNA integrity, extraction efficiency, and procedural simplicity [14].
Finally, in resource-limited settings, the choice of method must balance performance with practicality. While the Spin-Column method performed best for detecting Clostridium perfringens, the Hotshot method was identified as a feasible, low-equipment alternative for on-site LAMP assays, albeit with lower sensitivity [47].
Detailed Experimental Protocols
To ensure reproducibility and provide a clear framework for method evaluation, this section outlines the core protocols for key experiments cited in this guide.
Protocol: SHIFT-SP Method Optimization
The SHIFT-SP protocol was developed by optimizing parameters for magnetic silica bead-based NA extraction [10].
- Sample Preparation: A known quantity of purified Mycobacterium smegmatis DNA (100 ng or 1000 ng) is spiked into Lysis Binding Buffer (LBB).
- Binding Optimization:
- pH: The binding efficiency is tested using LBB at pH 8.2 and pH 4.1. The lower pH (4.1) is found to significantly enhance DNA binding to the silica beads by reducing electrostatic repulsion.
- Mixing Mode: "Orbital shaking" is compared to a "tip-based" method, where the binding mix is aspirated and dispensed repeatedly using a pipette. The tip-based method achieves ~85% binding in 1 minute, far surpassing orbital shaking.
- Bead Quantity: For higher input DNA (1000 ng), increasing the bead volume from 10 µL to 30-50 µL raises binding efficiency from ~56% to over 92%.
- Washing: Beads are washed according to the standard VERSANT sample preparation protocol to remove impurities and chaotropic salts.
- Elution: Bound NA is eluted with an appropriate elution buffer. Factors such as temperature, pH, and duration are optimized for maximum elution efficiency.
- Quantification: DNA is quantified using a qPCR-based approach. To negate the PCR-inhibitory effects of guanidine in the LBB, samples are diluted 500-fold in 1X TE buffer before qPCR analysis [10].
Protocol: Comparing Automated DNA Extractors for Stool
This protocol evaluates the efficiency of automated nucleic acid extractors for microbiome studies [9].
- Sample Collection and Preparation: Human fecal samples are collected and immediately processed in an anaerobic chamber. One gram of stool is aliquoted into a DNA/RNA Shield Fecal Collection Tube containing preservation reagent and stored at -80°C until extraction. A mock microbial community standard is used as a control.
- Mechanical Lysis: Prior to automated extraction, samples (300 µL of fecal mixture, 75 µL of mock community) are homogenized using a bead-beating grinder at 6.0 m/s for 40 seconds. A parallel set of samples is processed without bead-beating for comparison.
- Automated DNA Extraction: Processed samples are loaded onto three automated systems: Bioer GenePure Pro, Promega Maxwell RSC 16, and ThermoFisher KingFisher Apex. Each system is operated according to the manufacturer's instructions using their proprietary kits.
- DNA Quantification and Quality Control:
- Concentration: Measured using a Qubit fluorometer with a dsDNA HS assay kit.
- Purity: Assessed using a NanoDrop One spectrophotometer.
- Downstream Analysis: Extracted DNA undergoes 16S rRNA gene amplicon sequencing (Illumina MiSeq). Data analysis includes inferring Amplicon Sequence Variants (ASVs), taxonomic classification, and calculating alpha- and beta-diversity metrics to evaluate the impact of the extraction method on microbiome composition [9].
The Scientist's Toolkit: Key Research Reagent Solutions
Table 3: Essential reagents and materials for nucleic acid extraction protocols.
| Reagent/Material | Function in the Protocol | Example Use Case |
|---|---|---|
| Magnetic Silica Beads | Solid phase for nucleic acid binding in the presence of chaotropic salts. | Core of the SHIFT-SP and other bead-based extraction methods [10]. |
| Lysis Binding Buffer (LBB) with Chaotropes | Disrupts cells, inactivates nucleases, and facilitates NA binding to silica. | Contains guanidine salts in the SHIFT-SP and Boom methods [10]. |
| DNA/RNA Shield Preservation Reagent | Stabilizes nucleic acids in samples immediately upon collection, preventing degradation. | Used for storage of fecal samples in microbiome studies prior to extraction [9]. |
| Proteinase K | Enzyme that digests proteins and nucleases, aiding in cell lysis and purifying NA. | Common component in lysis buffers for complex samples like stool and tissues. |
| Binding Matrix Solution | A suspension that captures nucleic acids after lysis, often used in column-based protocols. | Used in the manual FastDNA Spin Kit for Soil for DNA binding [9]. |
| Elution Buffer (e.g., TE Buffer) | A low-salt, slightly alkaline solution used to dissolve and preserve eluted nucleic acids. | Used to elute DNA from silica matrices and as a dilution buffer for qPCR [10]. |
Workflow and Pathway Visualizations
SHIFT-SP Optimization Workflow
The following diagram illustrates the key optimized steps in the SHIFT-SP nucleic acid extraction method.
Pitfall-Solution Relationship Diagram
This diagram maps the common pitfalls in nucleic acid extraction to their evidence-based solutions.
The fundamental challenge in nucleic acid extraction lies in efficiently breaking down complex cellular structures while preserving the integrity of the target molecules and minimizing co-purification of inhibitors. This guide objectively compares the performance of organic extraction methods against various solid-phase techniques—including spin columns and magnetic bead-based systems—for processing three particularly challenging sample types: Gram-positive bacteria, plant tissues, and forensic evidence. The rigid, multi-layered peptidoglycan cell wall of Gram-positive bacteria, the abundant polysaccharides and polyphenols in plant cells, and the degraded, inhibitor-rich nature of forensic samples each present unique obstacles that demand tailored extraction strategies [48] [49]. The selection of an appropriate method significantly impacts downstream applications, from the success of PCR and quantitative assays to the reliability of next-generation sequencing and microarray analyses [11] [15].
The ongoing methodological evolution reflects a shift from traditional organic techniques toward more amenable solid-phase protocols. This comparison provides researchers with a data-driven framework for selecting optimal nucleic acid extraction methods based on specific sample requirements, throughput needs, and desired output quality.
Methodological Comparison: Organic vs. Solid-Phase Extraction
Core Principles and Workflows
Organic Extraction relies on liquid-phase separation. Samples are homogenized in a phenol-chloroform mixture, and upon centrifugation, the aqueous phase containing nucleic acids is separated from the organic phase and interface, which contain denatured proteins and lipids. The nucleic acids are then recovered from the aqueous phase via alcohol precipitation [11] [49].
Solid-Phase Extraction utilizes a solid support to bind nucleic acids. In spin column methods, a silica membrane selectively binds DNA or RNA under high-salt conditions, impurities are washed away, and pure nucleic acids are eluted in a low-salt buffer [11] [50]. Magnetic bead methods function similarly, using silica-coated paramagnetic beads that are collected with a magnet through wash and elution steps [11] [21].
Performance Metrics Across Sample Types
Table 1: Comprehensive Method Comparison for Challenging Samples
| Sample Type | Extraction Method | Reported Yield | Purity (A260/A280) | Key Advantages | Key Limitations |
|---|---|---|---|---|---|
| Gram-Positive Bacteria | Organic (Phenol-Chloroform) | High (Baseline) | 1.8-2.0 [49] | Effective lysis, high yield [49] | Laborious, toxic reagents [11] |
| Spin Column | Variable (Low with incomplete lysis) [11] | 1.7-2.0 | Convenient, rapid [11] | Membrane clogging, low yield with tough cells [11] | |
| Bead Beating + Spin Column | >15-fold increase in L. lactis; >6-fold in E. faecium [48] | Acceptable (RIN >7 for RNA) [48] | Superior for tough cell walls [48] | Requires optimization, potential for shearing [48] | |
| Magnetic Beads (NiFe₂O₄) | High (Cost-effective) [21] | Suitable for downstream applications [21] | Amenable to automation, low cost [21] | Protocol optimization needed [21] | |
| Plant Materials | CTAB + Organic | High (Gold Standard) [49] | 1.8-2.0 (With PVP) [49] | Removes polysaccharides/polyphenols [49] | Time-consuming, multiple steps [49] |
| CTAB + Spin Column | High | 1.8-2.0 | Removes inhibitors, user-friendly [49] | Sample amount limitations [50] | |
| Magnetic Beads | High | Good Purity [51] | High-throughput, automatable [51] [50] | Bead loss risk, manual handling tedious [11] | |
| Forensic Evidence (Saliva) | Organic | High DNA yield [15] | Variable with co-isolation | Proven efficacy for DNA [11] | Poor miRNA recovery, hazardous [15] |
| Spin Column (DNA Kit) | 72.1 ng DNA (semen); 4.5 ng DNA (saliva) [52] | Good (With inhibitors) | Good DNA recovery, standardized [52] | Lower RNA co-recovery [52] | |
| Magnetic Bead (Protamine) | 65.2 ng DNA (semen); 6.4 ng DNA (saliva) [52] | Good | Excellent DNA/RNA co-recovery, preserves proteins [52] | Newer method, less validation [52] |
Table 2: Cost and Throughput Analysis
| Extraction Method | Cost per Sample (EUR) | Suitability for High-Throughput | Automation Potential | Hands-On Time |
|---|---|---|---|---|
| Organic Extraction | ~0.37 (Traditional) [21] | Low [11] | Difficult [11] | High [11] |
| Spin Column | ~1.34 (Commercial Kit) [21] | Medium (96-well format) [50] | Medium (Vacuum manifolds) [50] | Medium [11] |
| Magnetic Beads | ~0.19 (MNP-based protocols) [21] | High (384-well format) [50] | High [11] [50] | Low (when automated) [11] |
Sample-Specific Optimization Strategies
Gram-Positive Bacteria: Overcoming Resilient Cell Walls
The rigid, multi-layered peptidoglycan structure of Gram-positive bacterial cell walls presents a formidable barrier to efficient lysis. Experimental data demonstrates that mechanical disruption via bead beating significantly enhances nucleic acid recovery. An optimized protocol employing three cycles of glass bead beating yielded a greater than 15-fold increase in RNA from Lactococcus lactis and a more than 6-fold increase from Enterococcus faecium, while maintaining RNA integrity (RIN >7) [48]. This mechanical approach physically disrupts the tough cell walls, complementing the chemical lysis achieved by traditional organic extraction or kit-based buffers.
For plasmid DNA (pDNA) isolation from bacterial cells, magnetic nanoparticles (MNPs) present a cost-effective and efficient alternative. A 2025 study demonstrated that nickel ferrite (NiFe₂O₄) and its amine-functionalized form successfully isolated high-quality pDNA, with transformation efficiency into competent cells confirming the functional integrity of the extracted DNA [21]. The MNP-based method was notably cost-effective, at approximately 0.19 EUR per isolation, compared to 1.34 EUR for a commercial spin column kit and 0.37 EUR for traditional organic extraction [21].
Plant Materials: Managing Polysaccharides and Polyphenols
Plant tissues are notoriously challenging due to their high content of secondary metabolites, such as polysaccharides and polyphenols, which can co-precipitate with nucleic acids and inhibit downstream enzymatic reactions. The CTAB (cetyltrimethylammonium bromide) method, often combined with either organic extraction or spin column purification, remains the gold standard [49]. CTAB effectively complexes with polysaccharides and polyphenols in a high-salt buffer, allowing for their separation from nucleic acids.
Key optimization strategies for plant DNA extraction include:
- Addition of PVP (polyvinylpyrrolidone): PVP binds to and neutralizes polyphenols, preventing them from oxidizing and causing brown discoloration or degrading nucleic acids [49].
- High-salt concentrations: Using NaCl concentrations of 1.4M helps to prevent polysaccharides from co-precipitating with DNA [49].
- Beta-mercaptoethanol: This reducing agent helps to inhibit oxidizing agents and RNases, further protecting the target nucleic acids [49].
For high-throughput applications, such as the genotyping of medicinal herbs and seeds, magnetic bead-based automated extraction kits have shown excellent performance, yielding DNA with acceptable concentration and purity for subsequent nucleic acid amplification assays like Proofman-LMTIA [51].
Forensic Evidence: Maximizing Recovery from Trace and Mixed Samples
Forensic samples often involve minute quantities of biological material, degraded nucleic acids, and complex mixtures of body fluids. The critical requirement is to maximize recovery from these trace samples. A novel method using salmon protamine conjugated to magnetic beads demonstrated comparable DNA recovery to a commercial forensic kit (Prepfiler) from semen (65.2 ng vs. 72.1 ng) and saliva (6.4 ng vs. 4.5 ng), with the added advantage of simultaneously recovering RNA and preserving proteins for multi-omics analysis [52]. This non-destructive approach is particularly valuable for irreplaceable evidence.
For body fluid identification using microRNA (miRNA) biomarkers, the choice of extraction kit is paramount. Surprisingly, a dedicated miRNA isolation kit (miRNeasy) yielded relatively poor miRNA recovery from saliva. In contrast, a DNA extraction kit (AccuPrep Genomic DNA Extraction Kit) produced the highest nucleic acid yield and showed the lowest quantification cycle (Cq) values for miRNA targets, indicating superior detection sensitivity [15]. This finding underscores that kits not specifically designed for miRNA can sometimes be more effective for their co-isolation, a critical consideration for forensic workflows.
The Researcher's Toolkit: Essential Reagents and Materials
Table 3: Key Reagents and Materials for Nucleic Acid Extraction
| Reagent/Material | Function | Application Examples |
|---|---|---|
| Phenol-Chloroform [11] [49] | Organic denaturant for liquid-liquid separation of proteins from nucleic acids. | Standard organic extraction from blood, tissues, bacteria. |
| Silica Membranes/Magnetic Beads [11] [21] [50] | Solid-phase support that binds nucleic acids under high-salt conditions. | Spin columns; magnetic bead-based automated and manual kits. |
| CTAB (Cetyltrimethylammonium Bromide) [49] | Detergent that complexes with polysaccharides and polyphenols. | DNA extraction from plant tissues (e.g., leaves, seeds). |
| Proteinase K [49] | Broad-spectrum serine protease that digests proteins and inactivates nucleases. | Lysis of animal tissues, forensic stains, and bacterial cells. |
| PVP (Polyvinylpyrrolidone) [49] | Polymer that binds to and removes polyphenols. | Preventing oxidation in plant samples rich in polyphenols (e.g., tea, grapes). |
| Glass Beads (100-500 µm) [48] | Mechanical homogenization agent for disrupting tough cell walls. | Lysis of Gram-positive bacteria (e.g., L. lactis, E. faecium). |
| Magnetic Nanoparticles (e.g., NiFe₂O₄) [21] | Paramagnetic particles for solid-phase nucleic acid binding and separation. | Cost-effective plasmid and genomic DNA isolation from bacteria. |
| Salmine (Salmon Protamine) [52] | Arginine-rich protein that tightly binds DNA and RNA. | Novel forensic method for co-extracting DNA/RNA from trace evidence. |
| EDTA (Ethylenediaminetetraacetic acid) [53] [49] | Chelating agent that binds metal ions, inhibiting nuclease activity. | Standard component of lysis and storage buffers across most protocols. |
| Chaotropic Salts (e.g., Guanidine HCl) [11] | Disrupts cellular structures and promotes binding of nucleic acids to silica. | Found in lysis and binding buffers of solid-phase extraction kits. |
The optimal nucleic acid extraction method is contingent on the specific sample type, analytical goals, and operational constraints. Organic extraction remains a powerful, high-yield technique for complex samples but its use is increasingly limited by safety concerns, procedural complexity, and poor suitability for high-throughput automation [11].
Solid-phase methods have become the mainstream choice. Spin columns offer a robust balance of convenience, quality, and cost for routine processing [11] [50]. Magnetic bead-based systems excel in high-throughput and automated environments, providing superior scalability and lower per-sample costs, particularly for labs with significant sample volumes [21] [50].
For the most challenging samples, integrated strategies that combine mechanical lysis (e.g., bead beating) with optimized solid-phase chemistries consistently deliver the best results. The emerging trend toward multi-analyte recovery—simultaneously isolating DNA, RNA, and protein from a single sample—as demonstrated by novel protamine-based magnetic bead methods, points to the future of holistic sample analysis in life science research [52].
Nucleic acid extraction serves as the foundational first step in a vast array of molecular biology applications, from routine PCR to cutting-edge next-generation sequencing and diagnostic assay development [11] [7]. The selection of an appropriate extraction methodology directly influences experimental success, data integrity, and operational efficiency within research and drug development laboratories. This guide provides a rigorous comparative analysis of the three predominant nucleic acid extraction techniques—organic extraction, spin column-based extraction, and magnetic particle-based extraction—framed within the broader thesis of organic versus solid-phase extraction research. We present objective performance data, detailed experimental protocols, and comprehensive cost-benefit evaluations to inform researchers and scientists in making strategically sound methodological choices that align with their project requirements, throughput needs, and budgetary constraints.
The evolution from traditional liquid-phase organic extraction to contemporary solid-phase methods represents a significant shift in laboratory practice, offering distinct trade-offs between purity, throughput, cost, and technical demand [11] [54] [7]. Each method operates on distinct biochemical principles for cell lysis, nucleic acid separation, and purification, factors which subsequently dictate their suitability for different laboratory environments, from academic research settings to high-throughput clinical diagnostics and pharmaceutical development pipelines.
Methodologies at a Glance: Core Principles and Procedures
The following diagram illustrates the fundamental procedural workflows for the three primary extraction methods, highlighting key divergences in their operational steps.
Detailed Experimental Protocols
To ensure reproducibility and provide a clear basis for comparison, detailed protocols for each extraction method are outlined below. These protocols are adapted from standardized procedures used in comparative studies [11] [55] [7].
Organic Extraction Protocol
- Lysis: Homogenize the sample in a phenol-chloroform solution to dissolve cellular membranes and denature proteins.
- Phase Separation: Centrifuge the mixture. Proteins partition into the lower organic phase and interface, while nucleic acids remain in the upper aqueous phase at appropriate pH [11].
- Recovery: Carefully pipette the aqueous upper phase without disturbing the organic phase or interface to avoid protein contamination.
- Precipitation & Wash: Precipitate nucleic acids from the aqueous phase using ethanol or isopropanol. Pellet the nucleic acids via centrifugation, wash with 70% ethanol, and briefly air-dry [7].
- Resuspension: Dissolve the purified nucleic acid pellet in TE buffer or nuclease-free water for downstream applications [7].
Spin Column Extraction Protocol
- Lysis & Binding: Lyse the sample in a buffered solution containing a chaotropic salt (e.g., guanidinium isothiocyanate) and RNase inhibitors. Load the lysate onto a silica-membrane spin column and centrifuge. Nucleic acids bind to the silica in the presence of chaotropic salts [11] [7].
- Washing: Wash the membrane twice with an ethanol-containing buffer to remove salts, proteins, and other contaminants. Centrifuge after each wash to remove the flow-through.
- Elution: Apply a low-salt elution buffer (e.g., TE buffer) or nuclease-free water to the center of the membrane, incubate for 1-5 minutes, and centrifuge to collect the purified nucleic acids [11].
Magnetic Bead Extraction Protocol
- Lysis & Binding: Lyse the sample as for spin columns. Add paramagnetic silica beads to the lysate and incubate to allow nucleic acids to bind to the bead surface [11] [54].
- Magnetic Separation: Apply an external magnetic field to immobilize the beads against the tube wall. Discard the supernatant containing impurities.
- Washing: While the beads are immobilized, add wash buffer to resuspend and wash the beads. Re-apply the magnet and discard the supernatant. Repeat as needed.
- Elution: Resuspend the beads in elution buffer or nuclease-free water. The purified nucleic acids are released into solution and the beads are separated magnetically. The final supernatant contains the purified nucleic acids [11].
Comparative Performance and Cost-Benefit Analysis
Quantitative Method Comparison
The table below synthesizes experimental data and characteristic profiles for a direct comparison of the three core extraction methods [11] [56] [55].
Table 1: Comprehensive Comparison of Nucleic Acid Extraction Methods
| Evaluation Metric | Organic Extraction | Spin Column Extraction | Magnetic Bead Extraction |
|---|---|---|---|
| Relative Reagent Cost | Low (Basic chemicals) | Moderate | Moderate to High |
| Hands-On Time | High (Labor-intensive) | Moderate | Low (Especially when automated) |
| Ease of Automation | Low | Moderate (Centrifugation required) | High (Inherently automation-friendly) |
| Throughput Potential | Low | Moderate | High |
| Typical Nucleic Acid Yield | High | Moderate to High | Moderate to High |
| Nucleic Acid Purity | High (Gold standard) [11] | High | High |
| Upfront Equipment Cost | Low (Requires centrifuge, fume hood) | Low to Moderate (Requires centrifuge or vacuum manifold) | High (Requires magnetic stands; automated systems cost $10,000–$70,000) [56] |
| Hazardous Waste | High (Toxic organic solvents) [11] | Low | Low |
| Key Advantages | • Well-established protocol• Effective for tough tissues• No specialized equipment needed | • Simple, standardized procedure• Kit-based convenience• Flexible for various sample types | • Amenable to high-throughput and automation• Rapid separation steps• No filter clogging issues [11] |
| Key Limitations | • Use of hazardous phenol-chloroform• Difficult to automate• Laborious for many samples [11] | • Membrane clogging risk with large or viscous samples• Incomplete lysis can lead to low yields [11] | • High capital cost for automation• Beads can be retained in viscous samples [11] |
Experimental Data from Comparative Studies
A 2025 study directly comparing DNA extraction methods from Dried Blood Spots (DBS) provides robust quantitative insights. The study evaluated three column-based kits (QIAamp DNA mini kit, High Pure PCR Template Preparation kit, DNeasy Blood & Tissue kit) and two in-house boiling methods (TE buffer, Chelex-100 resin) [55].
Key findings revealed that the Chelex boiling method yielded significantly higher DNA concentrations compared to all other methods when measured by qPCR targeting the ACTB gene. Among the column-based methods, the Roche High Pure kit showed significantly higher DNA concentrations than the other column kits as measured by spectrophotometry. The study also demonstrated that optimization, such as reducing the elution volume from 150 µL to 50 µL, significantly increased the final DNA concentration, a critical factor for downstream applications like qPCR [55].
Another study focusing on challenging museum specimen samples found that while different DNA extraction methods did not significantly differ in yield, the choice of library build method was crucial for data quality from degraded DNA, highlighting that the optimal workflow depends on the sample type and downstream application [57].
The Scientist's Toolkit: Essential Research Reagent Solutions
Successful nucleic acid extraction relies on a suite of specialized reagents and materials, each playing a critical role in the multi-step process of isolation and purification.
Table 2: Key Reagents and Materials for Nucleic Acid Extraction
| Item | Function in the Protocol |
|---|---|
| Lysis Buffer | Breaks open cells and nuclei, inactivating nucleases and releasing nucleic acids. Often contains detergents and salts. |
| Chaotropic Salts | Disrupts hydrogen bonding, making nucleic acids adhere to silica in solid-phase methods [7]. |
| Proteinase K | Digests and denatures proteins, removing histones and other cellular proteins from the nucleic acids. |
| Phenol-Chloroform | Organic solvent mixture used to denature and remove proteins during organic extraction [11]. |
| Silica Membrane/Beeds | The solid phase that selectively binds nucleic acids in the presence of chaotropic salts, separating them from other cellular components [11] [54]. |
| Wash Buffer | Typically an alcohol-based solution used to remove salts, metabolites, and other impurities from the bound nucleic acids without eluting them. |
| Elution Buffer | A low-salt buffer or nuclease-free water used to break the interaction between the nucleic acid and the solid phase, resulting in the final purified product. |
| RNase Inhibitors | Essential for RNA extraction to prevent degradation by ubiquitous RNases. |
| Magnetic Stand | A device that generates a magnetic field to immobilize magnetic beads during washing and elution steps. |
The choice of nucleic acid extraction method is a strategic decision that balances cost, time, quality, and scalability. Organic extraction remains a powerful, low-cost option for processing difficult samples where ultimate purity is desired and throughput is low, but its labor-intensive nature and hazardous waste stream are significant drawbacks [11]. Spin column-based methods offer an excellent balance of convenience, quality, and cost for most routine laboratory applications, making them a versatile and widely adopted solution [11]. Magnetic particle-based methods represent the pinnacle of efficiency for high-throughput laboratories, dramatically reducing hands-on time and enabling full automation, albeit with a higher initial investment in equipment [11] [56].
Researchers and drug development professionals should base their selection on a clear assessment of their primary needs. For labs processing hundreds to thousands of samples weekly, the capital investment in magnetic bead automation is readily justified by the savings in personnel time and the improvements in reproducibility. For smaller-scale or resource-constrained settings, optimized spin column or even simple boiling/Chelex methods can provide high-quality DNA suitable for many downstream applications, including qPCR [55]. Ultimately, there is no universally superior method; the optimal technique is the one that most effectively and economically meets the specific demands of the scientific question and operational context.
Within the broader scope of comparing organic versus solid-phase nucleic acid extraction methods, the critical subsequent step of quality control cannot be overlooked. The choice of extraction technique—be it the classic organic phenol-chloroform method or various solid-phase approaches—directly impacts the quantity, quality, and purity of the recovered nucleic acids, which in turn influences the accuracy of their quantification [11]. Accurate quantification is paramount for the success of downstream applications, including qPCR, sequencing, and genotyping [58] [59]. This guide objectively compares three principal quantification techniques—UV spectrophotometry, fluorometry, and PCR amplification efficiency—by examining their performance characteristics, supported by experimental data from controlled studies.
Technical Principles and Comparison
The performance disparities between quantification methods stem from their fundamental operating principles. UV spectrophotometry relies on the absorbance of ultraviolet light at 260 nm by the heterocyclic rings of nucleotides. While simple and fast, this method cannot distinguish between DNA, RNA, and free nucleotides, and is highly susceptible to interference from common contaminants like salts or proteins that absorb at nearby wavelengths [58] [59].
In contrast, fluorometry utilizes dyes that fluoresce only when specifically bound to double-stranded DNA. This binding-specific mechanism makes fluorometry highly selective for the target molecule (e.g., dsDNA over RNA or contaminants), resulting in significantly higher accuracy for low-concentration samples and those with impurities [58] [60].
PCR amplification efficiency is not a direct quantification method per se, but a measure of how effectively a qPCR reaction amplifies its target. Efficiency (E) is calculated from a standard curve using the formula (E = 10^{(-1/slope)} - 1), with an ideal value of 1 (or 100%) [61]. This method specifically quantifies amplifiable DNA, providing the most relevant concentration data for downstream PCR-based applications.
Table 1: Core Principles and Characteristics of Nucleic Acid Quantification Methods.
| Method | Fundamental Principle | Measured Entity | Key Distinguishing Feature |
|---|---|---|---|
| UV Spectrophotometry | Absorbance of UV light at 260 nm [58] | Total nucleic acids (dsDNA, ssDNA, RNA) and free nucleotides [59] | Cannot discriminate between nucleic acid types; affected by contaminants [58] |
| Fluorometry | Fluorescence emission from dyes upon binding to specific nucleic acid structures [58] | Typically double-stranded DNA (with specific dyes) [60] | High specificity for target molecule; minimal interference from contaminants [58] |
| PCR Amplification Efficiency | Exponential amplification of target sequences in real-time [58] | Amplifiable template DNA [61] | Measures only sequences that will amplify in a PCR; highly sequence-specific [58] |
Performance Data and Experimental Evidence
A 2021 study directly compared DNA quantification from different sample types using both spectrophotometry (NanoDrop) and fluorometry (Qubit). The results, summarized in Table 2, reveal a consistent and significant overestimation of DNA concentration by spectrophotometry across all sample types, especially in processed samples like FFPE blocks and FNAC smears [62].
Table 2: Mean DNA Yield from Different Sample Types as Measured by Fluorometry vs. Spectrophotometry [62].
| Sample Type | Mean DNA Yield (Fluorometry) | Mean DNA Yield (Spectrophotometry) | Approximate Overestimation by Spectrophotometry |
|---|---|---|---|
| Peripheral Blood | 10.99 ng/µL | 29.76 ng/µL | 2.7x |
| FFPE Core Biopsy | 1.9 ng/µL | 69.9 ng/µL | 36.8x |
| FNAC Smear | 3.3 ng/µL | 119.9 ng/µL | 35.3x |
The study concluded that "DNA estimation by fluorometry is more accurate and precise than spectrophotometry" for these biologically relevant samples [62]. Gel electrophoresis confirmed that DNA from FFPE and FNAC samples was fragmented, which contributes to the inflation of spectrophotometric readings that count all DNA fragments and free nucleotides [62].
Impact on Quantitative PCR (qPCR) Results
The choice of quantification method has a direct and measurable impact on the analytical results of qPCR. A study on genetically modified maize quantification demonstrated that using spectrophotometric data could lead to significant inaccuracies. When DNA was intentionally degraded, spectrophotometry (A260) persistently reported higher concentrations than fluorometry (Picogreen). Using these inflated concentrations to normalize qPCR reactions led to an overestimation of the % GM content in the samples. The study found that "results normalized with Picogreen dye quantification were closer to the certified GM value," highlighting that fluorometric quantification provides a more reliable basis for downstream molecular analysis [63].
Variability in PCR Efficiency Estimation
The precision of PCR efficiency estimation itself is highly dependent on robust experimental design. Research investigating this variability found that the estimated PCR efficiency can differ significantly across different qPCR instruments. A Monte Carlo simulation revealed that the uncertainty in efficiency estimation could be as large as 42.5% (95% confidence interval) when a standard curve with only a single qPCR replicate is used. To achieve precise efficiency estimates, the study recommends: 1) generating one robust standard curve with at least 3–4 qPCR replicates at each concentration, 2) recognizing that efficiency is instrument-dependent but stable on a single platform, and 3) using a larger volume when constructing serial dilution series to reduce sampling error [61].
Experimental Protocols for Method Comparison
For researchers seeking to validate or compare these methods in their own laboratories, the following core protocols, synthesized from the cited literature, can serve as a guide.
- Sample Preparation: For FFPE blocks, take 3-4 shavings of 10-micron thickness from blocks with marked tumor areas. For stained FNAC smears, decover using xylene, then decolorize with 0.5% acid-alcohol.
- DNA Extraction: Use a commercial silica-membrane based kit (e.g., QIAamp DNA FFPE tissue kit). For smear scrapings, add a proteinase K incubation step (2-4 hours at 56°C).
- DNA Elution: Elute DNA in 40-100 µL of the provided elution buffer.
- Quantification: Quantify the same eluate using both a fluorometer (e.g., Quantus Fluorometer) and a UV-vis spectrophotometer (e.g., QIAxpert). Record concentrations and A260/280 ratios.
- Quality Assessment: Perform gel electrophoresis (1.5% agarose) to visualize DNA integrity.
- Sample Treatment: Subject extracted genomic DNA (e.g., from certified reference material) to a degradation time-course (e.g., heat at 95°C for 0-60 seconds).
- Parallel Quantification: Quantify intact and degraded samples using both spectrophotometry (A260) and a fluorometric dye (e.g., Picogreen).
- qPCR Normalization: Prepare qPCR reactions for a target gene (e.g., GM trait) and an endogenous control gene, normalizing input DNA using the concentrations derived from each quantification method.
- Analysis: Calculate the % GM content from the qPCR results (Cq values) for each normalization approach and compare against the known certified value.
Visualization of Workflow and Decision Logic
The following diagram illustrates the typical experimental workflow for comparing quantification methods and the logical factors influencing choice.
Research Reagent Solutions
The following table lists key reagents and kits commonly used for nucleic acid quantification, as featured in the cited experimental data.
Table 3: Essential Reagents and Kits for Nucleic Acid Quantification.
| Product Name | Type/Method | Primary Function | Key Feature / Evidence |
|---|---|---|---|
| QIAamp DNA FFPE Tissue Kit (Qiagen) [62] | Solid-Phase Extraction | DNA purification from formalin-fixed, paraffin-embedded tissue and smears. | Used in study showing high DNA yield from FNAC smears [62]. |
| Quantus Fluorometer (Promega) [62] | Fluorometry | Quantification of dsDNA using fluorescent dyes. | Provided accurate DNA quantification from blood, FFPE, and FNAC samples vs. spectrophotometry [62]. |
| Qubit dsDNA HS Assay Kit (Thermo Fisher) [64] [60] | Fluorometry | Highly specific quantification of dsDNA in low-concentration samples. | Consistently provided more accurate results than spectrophotometry for degraded and low-concentration samples [60]. |
| NanoDrop Spectrophotometer (Thermo Fisher) [62] [60] | UV Spectrophotometry | Rapid quantification and purity assessment (A260/280) of nucleic acids. | Tends to overestimate DNA concentration compared to fluorometric methods [62] [60]. |
| PicoGreen dsDNA Quantitation Reagent (Invitrogen) [63] | Fluorometry | Fluorescent dye for sensitive detection of dsDNA. | Provided more reliable qPCR normalization for GM content than A260 [63]. |
Head-to-Head Validation: Comparative Analysis of Extraction Performance and Clinical Utility
The field of nucleic acid extraction has undergone a significant transformation, moving from traditional organic-based methods to sophisticated solid-phase techniques. This evolution has been driven by the increasing demands of molecular diagnostics, which require high-quality DNA and RNA for sensitive downstream applications such as PCR, sequencing, and hybridization assays [7]. The core thesis of this comparison guide is that while organic extraction methods established the foundation for nucleic acid isolation, solid-phase techniques—particularly magnetic bead-based systems—demonstrate superior performance in direct comparisons of yield, purity, and inhibitor resistance, making them more suitable for modern research and clinical applications.
The fundamental process of nucleic acid extraction consists of five critical steps: cell lysis, lysate clearing, nucleic acid binding, washing, and elution [65]. It is in the implementation of these steps that organic and solid-phase methods diverge significantly, leading to measurable differences in performance outcomes. Organic methods rely on phase separation using toxic phenol-chloroform mixtures, while solid-phase methods utilize binding matrices such as silica membranes or magnetic beads under specific buffer conditions [7] [65]. As molecular diagnostics continue to advance, with applications ranging from pathogen detection to oncology screening, the choice of extraction methodology has become increasingly impactful on the success and reliability of downstream results.
Methodological Approaches in Comparison Studies
Organic Extraction Methods: Traditional organic extraction employs phenol-chloroform mixtures to separate nucleic acids from proteins and other cellular components through phase separation. After centrifugation, the aqueous phase containing DNA is recovered and precipitated with alcohol [7]. This method, while effective for high molecular weight DNA isolation, involves toxic, caustic, and flammable chemicals and requires significant hands-on time [7] [66].
Solid-Phase Extraction Methods: These methods utilize binding matrices such as silica membranes, columns, or magnetic beads to capture nucleic acids in the presence of chaotropic salts. The bound nucleic acids are washed and then eluted in low-salt buffers [7] [65]. The magnetic bead-based approach represents an advanced form of solid-phase extraction where nucleic acids bind to silica-coated magnetic beads, enabling automation and higher throughput [7] [10].
Experimental Designs for Direct Comparison
Rigorous comparison studies implement side-by-side evaluations of these extraction methods using standardized samples and downstream analytical techniques. Typical experimental approaches include:
Standardized Sample Preparation: Studies often use spiked samples with known concentrations of cultured bacteria (e.g., Escherichia coli, Staphylococcus aureus) or viral particles in various matrices (whole blood, fecal samples, cervical swabs) to control input material [39] [67] [66].
Inhibitor Resistance Testing: Researchers systematically introduce PCR inhibitors such as hemoglobin at increasing concentrations to evaluate the robustness of each extraction method [39].
Downstream Application Assessment: Extracted nucleic acids are evaluated in relevant downstream applications including PCR, qPCR, LAMP assays, and sequencing to determine functional performance [47] [66] [67].
Quantitative Measurements: Yield is quantified using spectrophotometry (NanoDrop) or fluorometry (Qubit), while purity is assessed through absorbance ratios (A260/A280 and A260/A230) and fragment analysis [66] [67].
Comparative Performance Data Analysis
Yield and Purity Metrics
Multiple studies have demonstrated significant differences in DNA yield and purity between extraction methods. In a comprehensive comparison of six DNA extraction methods for long-read sequencing, the Quick-DNA HMW MagBead Kit (magnetic bead-based) yielded the highest quality of pure high molecular weight DNA, outperforming other methods including traditional organic extraction [66]. Similarly, a 2025 study on sepsis pathogen detection found that magnetic bead-based methods (K-SL DNA Extraction Kit and GraBon system) provided superior DNA yields from whole blood samples compared to the column-based QIAamp DNA Blood Mini Kit [67].
Table 1: DNA Yield and Purity Across Extraction Methods
| Extraction Method | Sample Type | Average DNA Yield | Purity (A260/A280) | Study |
|---|---|---|---|---|
| Magnetic Bead (Quick-DNA HMW MagBead) | Bacterial mock community | High | ~1.8 | [66] |
| Magnetic Bead (K-SL DNA Extraction Kit) | Whole blood | 77.5% accuracy for E. coli | Not specified | [67] |
| Magnetic Bead (GraBon) | Whole blood | 76.5% accuracy for E. coli | Not specified | [67] |
| Column-based (QIAamp DNA Blood Mini) | Whole blood | 65.0% accuracy for E. coli | Not specified | [67] |
| Boiling method | Cervical swab | 10.02% detection rate | Susceptible to inhibitors | [39] |
| Phenol-Chloroform | Bacterial cells | High molecular weight | Potential organic contamination | [7] [66] |
Inhibitor Resistance Capabilities
Inhibitor resistance is a critical performance differentiator, particularly for clinical samples containing hemoglobin, bile salts, or other PCR inhibitors. A 2025 study comparing boiling versus magnetic bead extraction for HPV detection demonstrated striking differences in anti-interference capabilities [39]. The magnetic bead method successfully detected HPV positive controls even at hemoglobin concentrations of 60 g/L, while the boiling method failed at concentrations exceeding 30 g/L [39].
Table 2: Inhibitor Resistance Comparison
| Extraction Method | Inhibitor Type | Maximum Tolerance Level | Impact on Detection | Study |
|---|---|---|---|---|
| Boiling method | Hemoglobin | 20 g/L | Complete inhibition at 30 g/L | [39] |
| Magnetic bead method | Hemoglobin | 60 g/L | No inhibition observed | [39] |
| Magnetic bead method | Blood components | High | 77.5% detection accuracy in whole blood | [67] |
| Column-based | Blood components | Moderate | 65.0% detection accuracy in whole blood | [67] |
Downstream Application Performance
The true test of nucleic acid extraction efficiency lies in the performance of downstream applications. In a study evaluating DNA extraction methods for Clostridium perfringens detection, spin-column and magnetic bead methods yielded DNA of higher purity and quality that performed well in both LAMP and PCR assays [47]. For advanced sequencing applications, the quality of extracted DNA directly impacted Nanopore sequencing results, with the magnetic bead-based Quick-DNA HMW MagBead Kit enabling accurate detection of almost all bacterial species in a complex mock community [66].
Technological Advancements in Solid-Phase Extraction
Magnetic Bead Technology Innovations
Recent advancements in magnetic bead technology have significantly improved extraction efficiency. The SHIFT-SP (Silica bead based High yield Fast Tip based Sample Prep) method, developed in 2025, achieves nearly complete nucleic acid extraction within 6-7 minutes by optimizing factors such as pH during binding, bead movement mechanics, and elution conditions [10]. This method demonstrated that binding buffer at pH 4.1 resulted in 98.2% DNA binding within 10 minutes, compared to 84.3% binding at pH 8.6 [10]. Furthermore, innovative "tip-based" mixing achieved ~85% DNA binding within just 1 minute, compared to ~61% binding with conventional orbital shaking methods [10].
Automation and Throughput Considerations
Automation represents another significant advantage of magnetic bead-based systems. The GraBon automated platform, which utilizes the same reagents as the manual K-SL DNA Extraction Kit, demonstrated superior consistency and higher throughput for processing clinical samples [67]. This automation potential stems from the "mobile solid phase" nature of magnetic beads, which can be completely resuspended during wash steps to enhance contaminant removal without centrifugation or vacuum filtration [65].
Research Reagent Solutions Toolkit
Table 3: Essential Materials for Nucleic Acid Extraction Research
| Reagent/Kit | Primary Function | Application Context |
|---|---|---|
| Phenol-Chloroform Mixture | Organic phase separation for nucleic acid purification | Traditional method for high molecular weight DNA isolation [7] |
| Guanidine Hydrochloride | Chaotropic salt for cell lysis and nuclease deactivation | Facilitates nucleic acid binding to silica in solid-phase methods [65] |
| Silica-Coated Magnetic Beads | Solid-phase matrix for nucleic acid binding | Enable automated extraction and inhibitor removal [7] [10] |
| Proteinase K | Enzymatic digestion of proteins | Enhances cell lysis, particularly for gram-positive bacteria [65] |
| RNase A | Ribonuclease for RNA contamination removal | Optional addition to elution buffer for DNA-specific purification [65] |
| Ethanol/Isopropanol | Nucleic acid precipitation and washing | Used in both organic and solid-phase methods for contaminant removal [7] [65] |
The comprehensive analysis of direct comparison studies clearly demonstrates the superiority of solid-phase extraction methods, particularly magnetic bead-based technologies, over traditional organic approaches in most contemporary applications. The performance advantages in yield, purity, and inhibitor resistance make solid-phase methods particularly suitable for clinical diagnostics where reliability and consistency are paramount.
While organic extraction retains value for specific applications requiring very high molecular weight DNA, the risks associated with toxic chemicals and the labor-intensive nature limit its utility in high-throughput settings [7] [66]. The continuing innovation in magnetic bead technology, including optimized binding conditions and automated platforms, suggests further performance improvements are likely [10] [67]. Researchers should select extraction methodologies based on their specific sample types, downstream applications, and throughput requirements, with magnetic bead-based solid-phase extraction representing the current gold standard for most molecular diagnostics and research applications.
The choice of nucleic acid extraction method is a critical pre-analytical step that significantly influences the sensitivity, specificity, and overall reliability of human papillomavirus (HPV) DNA detection in molecular diagnostics. Within the broader research context comparing organic versus solid-phase extraction techniques, this case study provides a detailed performance comparison between two common approaches: the traditional boiling method (an organic preparation) and the modern magnetic bead-based method (a solid-phase technique). As cervical cancer screening programs globally adopt HPV DNA testing as the primary screening method, the optimization of nucleic acid extraction has become increasingly important for achieving accurate clinical results [68]. The boiling method, which relies on heat-induced cell lysis and chemical clarification, offers simplicity and low cost, whereas magnetic bead methods utilize silica-coated magnetic particles to bind nucleic acids through solid-phase extraction principles, enabling automated purification and potential performance advantages [69] [39]. This evaluation across 17,179 clinical samples provides evidence-based insights to guide laboratory decision-making for HPV detection protocols.
Experimental Design and Methodologies
Sample Collection and Preparation
The study utilized cervical swab specimens collected from female participants following standardized procedures to ensure sample quality and consistency. Participants were advised to avoid vaginal medications and menstrual periods during specimen collection. Clinical physicians used a speculum to expose the cervix, removed excess secretions, and collected exfoliated cells by inserting a cervical brush approximately 1 cm into the cervical canal and rotating it clockwise five times. The brush was then placed in preservation solution and transported to the laboratory for testing [69] [39]. This standardized collection protocol minimized pre-analytical variables that could affect downstream nucleic acid extraction and HPV detection efficiency.
Nucleic Acid Extraction Protocols
Boiling Method Protocol
The boiling-based DNA extraction procedure was performed as follows:
- The specimen tube was thoroughly mixed, and 300 µL of sample was transferred to a 1.5 mL Eppendorf tube.
- The tube was centrifuged at 14,000 rpm for 3 minutes, and the supernatant was discarded.
- 200 µL of nucleic acid extraction reagent (mainly consisting of CheLex 100; Tellgen Corporation, China) was added to the pellet and mixed thoroughly.
- The mixture was incubated in a 100°C metal bath for 15 minutes.
- The tube was centrifuged again at 14,000 rpm for 5 minutes.
- Finally, 5 µL of the supernatant was added to the corresponding PCR reaction tubes [69] [39].
Magnetic Bead Method Protocol
The magnetic beads-based DNA extraction (qEx-DNA/RNA virus T183, Tianlong Corporation, China) procedure included:
- A 300 µL sample was loaded into the extraction plate.
- The entire procedure was run automatically on the PANA 9600 s instrument (Tianlong Corporation, China).
- The automated protocol consisted of four sequential steps: lysis, magnetic attraction, washing, and elution.
- Finally, 5 µL of the eluate was transferred into the corresponding PCR reaction tubes [69] [39].
HPV DNA Genotyping Detection
HPV testing was performed using the Tellgenplex HPV27 DNA genotyping Test system (Tellgen Corporation, China). The experimental procedure included PCR amplification, hybridization, and fluorescence detection (Luminex 200TM, Thermo Fisher). The test detected 27 HPV genotypes: low-risk (LR) HPV types (6, 11, 40, 42, 43, 44, 55, 61, 81, 83) and high-risk (HR) HPV types (16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 82). Phycoerythrin fluorescence values greater than 150 were considered positive, while values less than 150 indicated negative results [69] [39].
Assessment of Anti-Hemoglobin Interference
Hemoglobin (Hb) has been shown to exert significant inhibitory effects during PCR amplification. To evaluate the anti-interference capabilities of both extraction methods:
- An EDTA-anticoagulated whole blood specimen with a hemoglobin level of 120 g/L was collected.
- The specimen was diluted with distilled water to concentrations of 120, 100, 80, 60, 40, 30, 20, 16, 12, 8, 4, and 0 g/L.
- Positive control specimen (including HPV genotypes 16 and 18) was mixed with the diluted whole blood specimens in a 1:1 ratio.
- The final hemoglobin concentrations in the HPV positive control were 60, 50, 40, 30, 20, 15, 10, 8, 6, 4, 2, and 0 g/L.
- The 300 µL mixed specimens underwent parallel nucleic acid extraction using both boiling and magnetic bead methods.
- Each sample was tested for HPV-DNA genotyping three times, and phycoerythrin fluorescence values were recorded [69] [39].
Comparative DNA Extraction Method Assessment
The study employed two complementary approaches to compare extraction method performance:
Paired Small-Sample Comparison: A total of 639 specimens for HPV testing were collected between December 19, 2024, and January 4, 2025. Both boiling and magnetic beads methods were applied simultaneously for each sample, and HPV results were recorded [69] [39].
Large-Scale Longitudinal Comparison: The study retrospectively collected HPV test results from the laboratory information system (LIS) before December 19, 2024, using the boiling method. Subsequently, specimens collected after January 4, 2025, were tested using the magnetic bead extraction method. The HPV detection rates of the two groups were compared across 16,540 cases [69] [39].
Statistical Analysis
Statistical analyses were conducted using SPSS software (version 16.0; IBM Corporation, USA). Chi-square tests or Fisher's exact tests were applied when comparing HPV infection rates across different groups. McNemar test was employed to compare HPV detection rates between boiling and magnetic bead methods within the same population. The consistency between methods was evaluated using the Kappa value. When comparing phycoerythrin fluorescence values between methods, a t-test or non-parametric test was used, as appropriate. Statistical significance was set at p < 0.05 [69] [39].
Results and Performance Comparison
Anti-Hemoglobin Interference Capability
The simulated hemoglobin interference experiment demonstrated significant differences in interference resistance between the two nucleic acid extraction methods:
Table 1: Anti-Hemoglobin Interference Comparison
| Hemoglobin Concentration (g/L) | Boiling Method Result | Magnetic Bead Method Result |
|---|---|---|
| 20 | Positive | Positive |
| 30 | Negative | Positive |
| 40 | Negative | Positive |
| 50 | Negative | Positive |
| 60 | Negative | Positive |
For the boiling method, HPV positive control could be detected when hemoglobin concentration was 20 g/L; however, at 30 g/L, hemoglobin caused strong interference, resulting in negative HPV results. In contrast, even when the hemoglobin concentration reached 60 g/L, the magnetic bead method for nucleic acid extraction remained unaffected, and the HPV positive control was still detected [69] [39]. This demonstrates the superior anti-interference capability of the solid-phase magnetic bead approach compared to the organic boiling method, particularly relevant for cervical specimens that may contain blood contamination.
HPV Detection Rate Comparison
Table 2: HPV Detection Rates Across Different Sample Sizes
| Study Scale | Sample Size | Boiling Method Positive Rate | Magnetic Bead Method Positive Rate | P-value |
|---|---|---|---|---|
| Paired Small-Sample | 639 cases | 10.02% (64/639) | 20.66% (132/639) | P < 0.001 |
| Longitudinal Large-Sample | 16,540 cases | Data not shown | Significantly higher | P < 0.001 |
In the paired small-scale experiment (639 cases), the positive detection rate of HPV using the magnetic bead method was significantly higher than that of the boiling method, with positive rates of 20.66% and 10.02% respectively (P < 0.001). Additionally, the longitudinal large-scale analysis (16,540 cases) reached the same conclusion, confirming the superior detection capability of the magnetic bead method across a substantial sample size [69] [39].
The nucleic acid concentrations obtained by the two methods also differed significantly. The median nucleic acid concentrations were 0.91 ng/µL (interquartile range: 0.36-2.74) for the boiling method and 1.82 ng/µL (interquartile range: 0.69-5.63) for the magnetic bead method (Z = -6.840, P < 0.001, Wilcoxon test), indicating approximately double the extraction efficiency with the magnetic bead approach [69].
Cost-Benefit Analysis
Table 3: Cost-Benefit Comparison of Extraction Methods
| Parameter | Boiling Method | Magnetic Bead Method | Change |
|---|---|---|---|
| Cost per test | Baseline | +13.14% | Increase |
| HPV Detection Rate | Baseline | +106.19% | Increase |
| Cost-Effectiveness | Reference | Highly improved | Superior |
Compared with the boiling method, the cost of the magnetic bead method increased by 13.14%; however, the detection rate of HPV increased by 106.19%, demonstrating highly favorable cost-effectiveness for the magnetic bead approach [69] [39]. This cost-benefit profile makes magnetic bead extraction particularly advantageous for large-scale screening programs where maximizing detection sensitivity is clinically important.
Discussion
Technical and Performance Advantages
The significant performance differences between boiling and magnetic bead extraction methods can be attributed to their fundamental technical principles. The boiling method, while simple and inexpensive, provides limited purification from PCR inhibitors such as hemoglobin, which is particularly problematic for cervical specimens that may contain blood contamination [69] [39]. In contrast, magnetic bead technology employs a multi-step purification process that effectively removes inhibitors through lysis, binding, washing, and elution stages, resulting in higher quality DNA and improved PCR amplification efficiency [70].
The superior nucleic acid yield and purity obtained with magnetic bead extraction (approximately double the concentration compared to boiling) directly translates to enhanced detection sensitivity for HPV genotypes. This is particularly important for detecting low viral loads or in samples with suboptimal cellularity. The automated nature of magnetic bead systems also reduces manual handling errors and improves reproducibility, crucial factors for large-scale screening programs [69] [70].
Implications for Cervical Cancer Screening
The findings from this extensive case study have significant implications for cervical cancer screening programs, particularly in light of the World Health Organization's global strategy to eliminate cervical cancer through the 90-70-90 targets for 2030. This strategy includes screening 70% of women with high-performance tests by ages 35 and 45 [71] [68]. The demonstrated 106.19% improvement in detection rates with magnetic bead extraction, coupled with its robust performance in the presence of potential inhibitors like hemoglobin, positions this solid-phase extraction method as a superior choice for achieving the sensitivity requirements of population-based screening.
While the boiling method may still offer utility in resource-limited settings due to its lower cost and technical simplicity, the modest 13.14% cost increase for magnetic bead extraction is substantially outweighed by its dramatic improvement in detection capability. Furthermore, the automation potential of magnetic bead systems enables higher throughput testing, reducing labor requirements and improving turnaround times for screening programs [69] [70].
Integration with Broader Nucleic Acid Extraction Research
This case study contributes valuable insights to the broader research discourse comparing organic versus solid-phase extraction methodologies. While organic methods like boiling offer simplicity, solid-phase techniques like magnetic beads consistently demonstrate superior performance in nucleic acid purification efficiency, inhibitor removal, and automation compatibility [41] [72]. Recent advancements in solid-phase extraction materials, including acid-activated bentonite and optimized sorbent combinations, continue to enhance nucleic acid recovery rates and purity [41] [72].
The principles demonstrated in this HPV detection case study have applicability across molecular diagnostics, including infectious disease testing, oncogenetics, and personalized medicine. As molecular assays continue to evolve toward higher sensitivity requirements and lower target detection thresholds, the importance of optimized nucleic acid extraction will only increase.
The Scientist's Toolkit: Essential Research Reagents
Table 4: Key Research Reagents and Solutions for Nucleic Acid Extraction
| Reagent/Solution | Function | Application Context |
|---|---|---|
| CheLex 100 Resin | Chelating agent that binds metal ions, protecting DNA during heat denaturation | Boiling method nucleic acid extraction |
| Silica-coated Magnetic Beads | Solid-phase matrix that binds nucleic acids under high salt conditions, enabling magnetic separation | Magnetic bead-based nucleic acid purification |
| Lysis Buffer | Disrupts cell membranes and nuclear envelopes to release nucleic acids | Initial step in both extraction methods |
| Wash Buffer | Removes proteins, inhibitors, and contaminants while retaining bound nucleic acids | Purification step in magnetic bead method |
| Elution Buffer | Low-salt solution that releases purified nucleic acids from solid-phase matrix | Final step in magnetic bead extraction |
| Proteinase K | Enzyme that digests proteins and nucleases, enhancing DNA yield and quality | Optional addition to lysis buffer |
| RNase A | Enzyme that degrades RNA to prevent interference with DNA analysis | Optional step for DNA-specific extraction |
Experimental Workflow Visualization
This comprehensive case study across 17,179 clinical samples demonstrates that the magnetic bead-based nucleic acid extraction technique exhibits superior anti-interference capabilities and significantly higher detection rates for HPV compared to the boiling method. The magnetic bead method maintained detection capability even at hemoglobin concentrations of 60 g/L, where the boiling method failed at 30 g/L. The dramatic 106.19% improvement in HPV detection rates, achieved with only a 13.14% cost increase, establishes the magnetic bead method as highly cost-effective for clinical HPV screening.
These findings strongly support the transition from traditional organic extraction methods to solid-phase magnetic bead technologies for HPV detection in cervical cancer screening programs. The automation compatibility, superior inhibitor resistance, and enhanced detection sensitivity of magnetic bead extraction align with the requirements of high-performance screening initiatives aimed at achieving the WHO's cervical cancer elimination targets. Further research should explore the application of these principles to other clinical contexts where nucleic acid extraction quality critically impacts diagnostic accuracy.
The choice of nucleic acid extraction method is a critical preliminary step in molecular biology, directly influencing the success and reliability of downstream analyses. Within the broader context of comparing organic and solid-phase extraction research, this guide objectively evaluates how different DNA extraction techniques impact performance in PCR, qPCR, next-generation sequencing (NGS), and microarray applications, supported by recent experimental data.
Nucleic acid extraction methodologies primarily fall into two categories: organic phase-separation (often liquid-liquid extraction using phenol-chloroform) and solid-phase extraction (SPE). While organic methods rely on phase separation to isolate nucleic acids, SPE techniques use a solid matrix, most commonly silica, to bind DNA or RNA in the presence of chaotropic salts, which is then washed and eluted. The transition towards SPE has been driven by demands for higher throughput, safety, and compatibility with automation. However, the performance of any method is ultimately validated by its efficiency in downstream applications, where factors such as yield, purity, and the absence of inhibitors are paramount [72].
Recent research continues to innovate in SPE. For instance, one study introduced an acid-activated bentonite (ASAB) as a novel solid-phase matrix, reporting a 3.95-fold increase in DNA recovery and a 6.3-fold improvement in RNA isolation efficiency compared to a commercial silica-based SPE kit. This demonstrates the ongoing evolution of SPE materials to overcome limitations like restricted surface area and suboptimal recovery [72].
Comparative Experimental Data on Extraction Methods
The following tables summarize key findings from recent studies that directly compared the performance of various DNA extraction methods in downstream applications.
Table 1: Comparison of DNA Extraction Methods from Dried Blood Spots (DBS) for qPCR Analysis [73]
| Extraction Method | Type | Mean ACTB DNA Concentration (ng/µL) via qPCR | Key Findings for Downstream qPCR |
|---|---|---|---|
| Chelex-100 Boiling | In-house, Physical | 12.46 | ► Significantly higher DNA yield (p < 0.0001).► Sufficient for TREC DNA detection, a key biomarker. |
| Roche High Pure Kit | Column-based, SPE | 3.41 | ► Best-performing commercial column kit.► Significantly higher yield than other columns. |
| QIAGEN DNeasy Kit | Column-based, SPE | 1.62 | ► Lower DNA recovery compared to Chelex and Roche. |
| QIAGEN QIAamp Kit | Column-based, SPE | 1.22 | ► Lower DNA recovery compared to Chelex and Roche. |
| TE Buffer Boiling | In-house, Physical | 2.36 | ► Moderate yield, lower than Chelex. |
Table 2: Comparison of DNA Extraction Methods from Ticks for Pathogen Detection via qPCR [64]
| Extraction Method | Type | DNA Purity (A260/280 Ratio) | Inhibition in qPCR | Key Findings for Downstream Pathogen Detection |
|---|---|---|---|---|
| Ammonium Hydroxide (Intact Tick) | In-house, Chemical | ~1.44 | 0/50 samples | ► As effective as commercial kits for qPCR.► Cheap, simple, and practical for large studies. |
| QIAGEN Blood & Tissue Kit | Column-based, SPE | 1.63 (Mean) | 0/50 samples | ► Provided the highest purity DNA.► Reliable amplification without inhibition. |
| Ammonium Hydroxide (Crushed Tick) | In-house, Chemical | ~1.44 | 9/50 samples | ► Crushing led to inhibitory substances in qPCR. |
| QIAGEN Mini Kit | Column-based, SPE | ~1.44 | 0/50 samples | ► Reliable amplification, but lower purity. |
Table 3: Performance of DNA Extraction Methods for LAMP Assay [47]
| Extraction Method | DNA Purity & Quality | Sensitivity in LAMP Assay | Practicality for Low-Resource Settings |
|---|---|---|---|
| Spin-Column (SC) | High | Highest detection capability | Less practical due to cost and equipment needs. |
| Magnetic Beads (MB) | High | High | Less practical due to cost and equipment needs. |
| Hotshot (HS) | Lower | Lower sensitivity | Most feasible and cost-effective for on-site use. |
Impact on Major Downstream Applications
PCR and Quantitative PCR (qPCR)
The sensitivity of (q)PCR to inhibitors makes DNA purity a critical factor. As shown in Table 2, the ammonium hydroxide hydrolysis method, while cost-effective, resulted in inhibitory substances when ticks were homogenized, highlighting how sample preparation intertwines with the extraction method [64].
The Chelex-100 boiling method has emerged as a robust, cost-effective contender for specific sample types like DBS. Its superior yield, as seen in Table 1, directly translated to successful detection of the low-abundance TREC biomarker, making it highly suitable for large-scale screening programs where cost and efficiency are crucial [73].
Furthermore, the choice of extraction method must align with the intended diagnostic assay. For field-based detection of Clostridium perfringens using LAMP, the Hotshot (HS) method, despite lower sensitivity, was identified as the most practical compromise for resource-limited settings [47].
Next-Generation Sequencing (NGS)
NGS places the highest demands on nucleic acid input, quality, and the preservation of complex population structures. Multi-template PCR, a fundamental step in many NGS library preparations, is highly susceptible to biases introduced during extraction and amplification. Sequence-specific variations in amplification efficiency can drastically skew abundance data, compromising the accuracy of quantitative results [74].
Advanced solid-phase extraction methods that maximize recovery are therefore crucial. The ASAB-based SPE system demonstrated superior efficiency in isolating viral RNA from clinical swabs and unstable microRNA, which is essential for sensitive NGS applications like transcriptomics [72]. The integrity of nucleic acids for NGS is often verified upstream using qPCR, underscoring the complementary nature of these technologies [75].
For RNA-Seq, the choice between qPCR and NGS depends on the research goal. While qPCR is excellent for targeted gene expression validation, RNA-Seq offers unbiased, hypothesis-free discovery of novel transcripts and isoforms, with a wider dynamic range for quantification [76].
Microarray Analysis
Although microarrays were not a primary focus of the recent search results, their performance is inherently linked to DNA quality and purity. Microarrays, which rely on the hybridization of fluorescently labeled nucleic acids to predefined probes, are sensitive to impurities that can cause high background noise or non-specific binding. The higher DNA purity obtained from methods like the QIAGEN Blood & Tissue Kit (Table 2) would be advantageous for microarray workflows to ensure specific hybridization and accurate signal detection [64].
Essential Research Reagent Solutions
The following table lists key reagents and materials referenced in the featured studies, which are essential for nucleic acid extraction and downstream analysis.
Table 4: Key Research Reagents and Their Functions
| Reagent / Material | Function in Nucleic Acid Workflow |
|---|---|
| Chelex-100 Resin | Chelating agent used in rapid, low-cost boiling methods to protect DNA from degradation and bind contaminants [73]. |
| Silica-Membrane Spin Columns | The core of many commercial SPE kits; nucleic acids bind to the silica membrane in the presence of chaotropic salts [73] [64]. |
| Ammonium Hydroxide | A simple, inexpensive alkaline reagent used for chemical lysis and DNA release from samples like ticks [64]. |
| Acid-Activated Bentonite (ASAB) | An innovative solid-phase matrix with enhanced surface area for improved nucleic acid binding and recovery [72]. |
| TaqMan Probes & Assays | Fluorogenic probes used in qPCR for highly specific and sensitive gene expression quantification and NGS result verification [75]. |
| Homobifunctional Imidoesters (HIs) | Cross-linking reagents used in novel SPE protocols for pH-dependent capture and release of nucleic acids [72]. |
Experimental Workflow and Decision Pathway
The diagram below illustrates a generalized workflow for comparing nucleic acid extraction methods and selecting the optimal one based on downstream application requirements.
Diagram 1: Workflow for comparing and selecting nucleic acid extraction methods based on final application needs. The process begins with defining the sample and research goals, proceeds through method comparison and evaluation of key performance metrics, and concludes with the selection of the optimal protocol for the intended downstream analysis.
The experimental data clearly demonstrates that no single nucleic acid extraction method is universally superior. The optimal choice is a careful balance between performance, practicality, and the specific demands of the downstream application.
- For high-sensitivity qPCR and low-abundance target detection in large-scale studies, cost-effective physical methods like Chelex-100 can outperform standard solid-phase columns [73].
- For complex applications like NGS, where representation bias and nucleic acid integrity are major concerns, advanced solid-phase methods that maximize recovery and minimize inhibitors, such as the ASAB-based system, show significant promise [74] [72].
- In resource-limited or field settings, simplicity and cost may override pure performance, making methods like ammonium hydroxide hydrolysis or Hotshot viable for diagnostic qPCR and LAMP assays [64] [47].
Therefore, researchers must contextualize their selection within the broader experimental workflow, validating that their chosen nucleic acid extraction method serves the ultimate goal of generating rigorous and reproducible data in their chosen downstream application.
Evaluating Scalability and Suitability for Point-of-Care Diagnostics (POC-Dx)
The "sample-to-answer" pipeline for Point-of-Care Diagnostics (POC-Dx) is a multi-stage process where nucleic acid extraction serves as the critical first step, profoundly influencing the sensitivity, speed, and ultimate success of the downstream diagnostic test. Within this context, extraction methodologies are often broadly categorized into organic extraction methods and solid-phase extraction methods, the latter of which includes column-based and magnetic bead-based protocols. Organic extraction, a traditional solution-based approach, relies on liquid-phase separation using chemicals like phenol and chloroform. In contrast, solid-phase extraction involves binding nucleic acids to a solid substrate, such as silica, under specific buffer conditions. The choice between these foundational approaches involves inherent trade-offs between purity, scalability, user-friendliness, and suitability for integration into automated, compact POC-Dx systems. This guide provides a comparative evaluation of these methods, focusing on their performance and scalability for decentralized diagnostic settings.
Comparative Analysis of Extraction Methods
The following tables summarize the core characteristics and performance data of the major nucleic acid extraction types, providing a objective comparison for researchers.
Table 1: Fundamental Characteristics of Nucleic Acid Extraction Methods
| Extraction Method | Core Technology / Chemistry | Key Advantages | Key Limitations / Challenges |
|---|---|---|---|
| Organic (Guanidinium Thiocyanate-Phenol-Chloroform) | Solution-based phase separation using phenol-chloroform mixtures [24]. | Considered a "gold standard" for purity; effective inactivation of nucleases [24]. | Use of toxic, hazardous chemicals; labor-intensive and time-consuming; difficult to automate; prone to carryover contamination [24] [77]. |
| Solid-Phase: Column-Based | Silica membrane in spin columns; nucleic acids bind in presence of chaotropic salts [24] [77]. | Eliminates toxic phenol/chloroform; more user-friendly than organic methods; commercial kits simplify the process [24]. | Susceptible to filter clogging; requires multiple centrifugation steps; potential for inconsistent yield [77]. |
| Solid-Phase: Magnetic Bead-Based | Silica-coated magnetic beads that bind nucleic acids in a magnetic field [24]. | Amenable to high-throughput and full automation; no centrifugation or vacuum steps required; enables integration into microfluidic POC devices [24] [78]. | Higher initial instrument cost; requires optimization of binding conditions; bead aggregation can be an issue [24]. |
Table 2: Performance Comparison of Automated & Manual Extraction Systems
A 2010 study compared several automated systems and one manual kit for their ability to recover DNA and RNA from respiratory pathogens, revealing performance variations [77]. The data below illustrates that recovery can be pathogen-specific, a critical consideration for diagnostic targets.
| Instrument/Kit (Core Technology) | Pathogen-Specific Performance Findings [77] | Approx. Protocol Time (min) [77] | Max. Extractions per Run [77] |
|---|---|---|---|
| Allprep (Manual, Glass Fiber Filter) | Baseline for comparison. | N/A (Manual) | N/A |
| KingFisher mL / easyMAG (Magnetic Beads) | Best for RNA viruses: 1- to 3-log wider linearity and 3- to 4-fold more RNA from influenza virus and respiratory syncytial virus [77]. | 20-45 | 15-24 |
| MagNA Pure Compact (Magnetic Beads) | Best for Gram-positive bacteria: >4-fold higher DNA recovery from S. pyogenes compared to other methods [77]. | 20 | 8 |
| Biorobot EZ1 (Magnetic Beads) | Equivalent recovery of L. pneumophila and adenovirus DNA to other automated systems [77]. | 20 | 6 |
Experimental Protocols for Key Comparisons
Protocol: Comparative Evaluation of Extraction Methods for Pathogen Recovery
This methodology is adapted from a study designed to compare the efficiency of six automated nucleic acid extraction systems and one manual kit for the recovery of respiratory pathogens [77].
- Sample Preparation: A pool of five respiratory pathogens (e.g., Streptococcus pyogenes, Legionella pneumophila, adenovirus, human influenza A virus, respiratory syncytial virus) is spiked into a human nasal wash matrix. Each specimen contains all five mixed pathogens. Bacteria are spiked at a concentration of approximately 5 × 10^6 cells, while viral cultures are titrated to a concentration that produces a Ct value around 25 in a downstream RT-PCR assay [77].
- Extraction Process: A set of 24 samples (100 µL each) is prepared for each extraction method being evaluated. The extractions are performed strictly following the manufacturers' instructions for each robotic system or manual kit. To monitor cross-contamination, a PCR-grade water control is processed in parallel with each batch. All samples are eluted in a standardized volume (e.g., 100 µL) of the elution buffer supplied by the manufacturer [77].
- Downstream Analysis & Data Collection: The extracted nucleic acids are analyzed using targeted real-time PCR or RT-PCR assays for each pathogen. The key performance metrics collected include:
- Recovery Efficiency: Calculated based on the Ct values, with a lower Ct indicating higher nucleic acid recovery.
- Linearity: Assessed by testing serial dilutions of the spiked samples.
- Reproducibility: Determined by performing extractions on three separate days.
- Inhibition: Checked by evaluating the PCR amplification curves of the samples [77].
Protocol: Evaluating DNA Extraction from Complex Processed Food Matrices
This protocol, derived from a 2025 study on food authenticity, outlines a rigorous comparison of DNA extraction methods from heavily processed samples, which share challenges with complex clinical samples like stool or sputum [79].
- Sample Matrix: Commercially marketed Chestnut rose juices and beverages are used as a model for a processed matrix. These samples have undergone mechanical, thermal, and chemical processing, which fragments and degrades DNA [79].
- Extraction Methods Compared: The study evaluates four distinct methods: a non-commercial CTAB-phenol-chloroform method, two commercial column-based kits (Plant Genomic DNA Kit), and one commercial magnetic bead-based kit (Magnetic Plant Genomic DNA Kit) [79].
- Assessment of DNA Quality and Quantity:
- Spectrophotometry: DNA concentration and purity (A260/A280 and A260/A230 ratios) are measured using a NanoDrop spectrophotometer.
- Gel Electrophoresis: Used to visually assess DNA integrity and fragmentation.
- Real-time PCR (qPCR): The amplifiability of the extracted DNA is tested using primers targeting a specific gene region (e.g., ITS2). This is the most critical metric for determining functional DNA quality [79].
- Evaluation of Practical Factors: The handling time, labor intensity, and cost per preparation for each method are also recorded and compared [79].
Workflow Visualization of Nucleic Acid Extraction Methods
The following diagram illustrates the key procedural steps and decision points for the primary nucleic acid extraction methods discussed, highlighting their differing complexities and suitability for automation.
The Scientist's Toolkit: Key Research Reagents & Materials
Successful evaluation and implementation of nucleic acid extraction methods, particularly for POC-Dx development, relies on a suite of essential reagents and materials.
Table 3: Essential Reagents and Materials for Nucleic Acid Extraction Research
| Reagent / Material | Function / Application in Research |
|---|---|
| Lysis Buffer (with Guanidinium Thiocyanate) | A powerful chaotropic agent that denatures proteins, inactivates nucleases, and disrupts cells and viruses, releasing nucleic acids while protecting them from degradation [24] [79]. |
| Silica-coated Magnetic Beads | The core of many modern automated and POC-Dx systems. Nucleic acids bind to the silica surface in the presence of chaotropic salts, allowing them to be magnetically separated and washed, then eluted in a clean buffer [24]. |
| Proteinase K | A broad-spectrum serine protease used to digest proteins and degrade nucleases, which significantly improves the purity and yield of extracted nucleic acids, especially from complex samples [79]. |
| Wash Buffers (typically Ethanol-based) | Used to remove salts, solvents, and other contaminants from the nucleic acids bound to the solid phase without causing them to elute, thereby increasing the final purity [24] [79]. |
| Nuclease-free Water or Low-Salt Elution Buffer | Used to resuspend or elute the purified nucleic acids after the final wash step. A low-ionic-strength environment promotes efficient release from the solid phase and is ideal for downstream applications like PCR [77] [79]. |
| PCR Inhibitors (as Control Spikes) | Substances like heparin, hemoglobin, or EDTA that are intentionally used during method development to test the robustness and purification efficiency of an extraction protocol against known inhibitors found in clinical samples [79]. |
The trajectory of nucleic acid extraction for POC-Dx is decisively shifting toward the full automation of solid-phase, particularly magnetic bead-based, methods. These systems are inherently more scalable and better suited for integration into compact, "sample-to-answer" diagnostic devices [78]. The future of extraction in POC-Dx will be shaped by its integration with advanced detection technologies like CRISPR-Cas, which demands pure, amplifiable nucleic acids for optimal function [80]. Furthermore, the incorporation of Machine Learning (ML) and Artificial Intelligence (AI) is poised to revolutionize POC-Dx platforms. ML algorithms can optimize sensor designs, interpret complex multiplexed results, and reduce subjective errors in test line reading, thereby enhancing overall diagnostic accuracy and reliability in decentralized settings [81]. Consequently, the choice of extraction method is no longer just about yield and purity, but about its compatibility with the integrated, connected, and intelligent POC-Dx systems of the future.
Conclusion
The choice between organic and solid-phase nucleic acid extraction is not a one-size-fits-all decision but depends on the specific requirements of the application. Organic methods, while historically the gold standard and cost-effective for basic research, involve hazardous chemicals and are less amenable to automation. Solid-phase methods, particularly silica-based columns and magnetic beads, offer superior safety, scalability, and ease of integration into automated, high-throughput workflows, making them indispensable for modern clinical diagnostics and drug development. Evidence from large-scale clinical validations demonstrates that while solid-phase methods may have a higher per-test cost, they provide significant advantages in detection sensitivity and inhibitor resistance, leading to more reliable results. Future directions will focus on further miniaturization, the development of novel sorbents, and the creation of fully integrated microfluidic systems that combine lysis, extraction, and amplification for next-generation point-of-care diagnostics. The ongoing evolution of extraction technologies will continue to underpin advancements in precision medicine, pathogen detection, and genetic research.
References
- 1. Organic Extraction Method | Thermo Fisher Scientific - ES [https://www.thermofisher.com/us/en/home/life-science/dna-rna-purification-analysis/organic-extraction.html]
- 2. A review of the modern principles and applications of solid ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9659506/]
- 3. Understanding and Improving Solid-Phase Extraction [https://www.chromatographyonline.com/view/understanding-and-improving-solid-phase-extraction-1]
- 4. Review Fifty years of solid-phase extraction in water analysis [https://www.sciencedirect.com/science/article/abs/pii/S0021967399011449]
- 5. Differences Between Solid-Phase Extraction (SPE) and ... [https://blog.organomation.com/blog/examining-the-differences-between-solid-phase-extraction-spe-and-solid-phase-micro-extraction-spme]
- 6. Research article Optimization of a solid-phase extraction ... [https://www.sciencedirect.com/science/article/pii/S2405844024166145]
- 7. Nucleic Acid Extraction Techniques - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC7122149/]
- 8. DNA Extraction Guide: Mastering 7 Essential Sample Types [https://www.thermofisher.com/blog/life-in-the-lab/dna-extraction-guide-mastering-7-essential-sample-types/]
- 9. A Comparison of Three Automated Nucleic Acid Extraction ... [https://www.mdpi.com/2076-2607/12/12/2417]
- 10. A rapid and high-yield method for nucleic acid extraction [https://www.nature.com/articles/s41598-025-95226-0]
- 11. The top pros and cons of different RNA extraction methods [https://lifescience.roche.com/global/en/article-listing/article/the-top-pros-and-cons-of-different-rna-extraction-methods.html]
- 12. A rapid and high-yield method for nucleic acid extraction [https://pubmed.ncbi.nlm.nih.gov/40216842/]
- 13. Nucleic Acid Extraction System 2025 Trends and Forecasts ... [https://www.archivemarketresearch.com/reports/nucleic-acid-extraction-system-479621]
- 14. Comparative evaluation of various DNA extraction methods ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11748555/]
- 15. Evaluation of extraction methods for co-isolation of nucleic ... [https://link.springer.com/article/10.1007/s00414-025-03641-9]
- 16. Chaotropic salts-based nucleic acid extraction coupled ... [https://www.sciencedirect.com/science/article/abs/pii/S0026265X25012111]
- 17. Silica-based solid-phase extraction of cross-linked nucleic ... [https://www.life-science-alliance.org/content/1/3/e201800088]
- 18. DNA extraction from complex matrices using magnetic ... [https://www.sciencedirect.com/science/article/abs/pii/S0003267025010256]
- 19. Comparison of DNA extraction methods for COVID-19 host ... [https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0287551]
- 20. An inhibitor-free, versatile, fast, and cheap precipitation-based ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11978010/]
- 21. Investigation and optimization of DNA isolation efficiency ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12274735/]
- 22. Nucleic acid extraction | Comparing DNA, RNA extraction ... [https://www.rocker.com.tw/en/application/nucleic_acid_extraction/]
- 23. Phenol-Chloroform, Silica Spin Column, Magnetic Beads [https://www.lunanano.com/post/comparing-nucleic-acid-purification-methods-phenol-chloroform-silica-spin-column-magnetic-beads?srsltid=AfmBOoqC2AVGxEjZ0DbKh456HoQjfLRffYSGQalFfurctj4oZr1pi4Od]
- 24. What are the different types of nucleic acid extraction? [https://frontlinegenomics.com/what-are-the-different-types-of-nucleic-acid-extraction/]
- 25. Comparison of Modified Manual Acid-Phenol Chloroform ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10275685/]
- 26. A Comparison of Three Automated Nucleic Acid Extraction ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11678849/]
- 27. A comparative analysis of extraction protocol performance ... [https://www.frontiersin.org/journals/ecology-and-evolution/articles/10.3389/fevo.2022.984056/full]
- 28. DNA purification: comparing different methods and techniques [https://www.integra-biosciences.com/united-states/en/blog/article/dna-purification-comparing-different-methods-and-techniques]
- 29. Acid guanidinium thiocyanate-phenol-chloroform extraction [https://en.wikipedia.org/wiki/Acid_guanidinium_thiocyanate-phenol-chloroform_extraction]
- 30. Single-step method of RNA isolation by acid guanidinium ... [https://pubmed.ncbi.nlm.nih.gov/2440339/]
- 31. How to Use Phenol-Chloroform for DNA Purification [https://www.thermofisher.com/us/en/home/references/protocols/nucleic-acid-purification-and-analysis/dna-extraction-protocols/phenol-chloroform-extraction.html]
- 32. A comparison of five DNA extraction methods from ... [https://www.sciencedirect.com/science/article/pii/S0379073823001809]
- 33. Top Solid Phase Extraction (SPE) Companies & How to ... [https://www.linkedin.com/pulse/top-solid-phase-extraction-spe-companies-how-za4fe/]
- 34. DNA Extraction Kits Compared: Spin Columns vs. Magnetic ... [https://synapse.patsnap.com/article/dna-extraction-kits-compared-spin-columns-vs-magnetic-beads]
- 35. Rapid DNA and RNA isolation from few or single cells ... [https://www.nature.com/articles/s41598-025-05770-y]
- 36. PCR Purification: Magnetic Beads vs Spin Columns [https://biotech-spain.com/en/articles/pcr-purification-magnetic-beads-vs-spin-columns-which-is-best-for-your-lab-/]
- 37. SPRI Beads vs Spin Columns [https://www.magbiogenomics.com/latest/post/magnetic-bead-dna-cleanup-protocols-optimizing-pcr-cleanups-with-highprep-vs-spin-columns?srsltid=AfmBOoqaGjyP2KQ7YAIRXGYbf8wQjtt_Zk0ZasHf01ZMyrYd4Kc0qcwr]
- 38. Solid Phase Extraction (SPE) System Analysis Report 2025 [https://www.archivemarketresearch.com/reports/solid-phase-extraction-spe-system-220248]
- 39. Comparison of boiling versus magnetic bead techniques in ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12613369/]
- 40. Optimization of solid phase extraction for simultaneous ... [https://www.frontiersin.org/journals/environmental-science/articles/10.3389/fenvs.2025.1539932/full]
- 41. Development of an Optimized Two-Step Solid-Phase ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12024844/]
- 42. BestProtocols: Cell Preparation for Flow Cytometry Protocols [https://www.thermofisher.com/us/en/home/references/protocols/cell-and-tissue-analysis/protocols/cell-preparation-flow-cytometery.html]
- 43. Collection, Storage, and Preparation of Human Blood Cells [https://pmc.ncbi.nlm.nih.gov/articles/PMC4524540/]
- 44. Ghost in the machine: What is automating your high- ... [https://www.rdworldonline.com/ghost-in-the-machine-what-is-automating-your-high-throughput-processes/]
- 45. The Benefits of Automation in High Throughput Screening [https://dispendix.com/blog/benefits-of-automation-in-high-throughput-screening]
- 46. Method development and validation for the quantification of ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7533261/]
- 47. Evaluation of different DNA extraction methods for the ... [https://www.sciencedirect.com/science/article/pii/S1056617125000844]
- 48. An optimised bead beating RNA extraction method for ... [https://www.sciencedirect.com/science/article/pii/S2950194625000731]
- 49. Advances in DNA Extraction: Methods, Improvement and ... [https://www.cd-genomics.com/blog/dna-extraction-methods-optimization-troubleshooting/]
- 50. DNA extraction | Definition, steps & methods | INTEGRA [https://www.integra-biosciences.com/united-states/en/blog/article/comprehensive-introduction-dna-extraction]
- 51. Comparison of Plant Genomic DNA Extraction Kits Using ... [https://ui.adsabs.harvard.edu/abs/2025PChAn..36.1118Y/abstract]
- 52. A novel method for simultaneous recovery of DNA, RNA ... [https://www.sciencedirect.com/science/article/abs/pii/S0379073825003093]
- 53. How to Work with Challenging or Limited Genomic ... [https://blog.omni-inc.com/blog/how-to-work-with-challenging-or-limited-genomic-samples-updated-for-2025]
- 54. Solid-phase extraction methods for nucleic acid separation. A review - PubMed [https://pubmed.ncbi.nlm.nih.gov/34453482/]
- 55. Comparison and Optimization of DNA Extraction Methods ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11932244/]
- 56. How Much Does DNA Extraction Equipment Cost? [https://www.excedr.com/blog/how-much-does-dna-extraction-equipment-cost]
- 57. a comparison of DNA extraction and library building methods [https://www.nature.com/articles/s41598-025-88443-0]
- 58. Choosing the Right Method for Nucleic Acid Quantitation [https://www.promega.com/resources/pubhub/choosing-the-right-method-for-nucleic-acid-quantitation/]
- 59. Nucleic Acid Quantification Methods: A Comprehensive ... [https://www.blue-raybio.com/en/blog/Nucleic-Acid-Quantification-Methods-A-Comprehensive-Guide]
- 60. Performance of Spectrophotometric and Fluorometric DNA ... [https://www.mdpi.com/2673-4532/3/3/25]
- 61. How good is a PCR efficiency estimate: Recommendations ... [https://www.sciencedirect.com/science/article/pii/S2214753515000169]
- 62. Comparison of Fluorometric and UV Spectrophotometric ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8672151/]
- 63. Comparison of fluorometric and spectrophotometric DNA ... [https://www.sciencedirect.com/science/article/abs/pii/S0956713508001837]
- 64. Making the best of a bad sample: Comparison of DNA ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12121741/]
- 65. DNA Purification | DNA Extraction Methods [https://www.promega.com/resources/guides/nucleic-acid-analysis/dna-purification/]
- 66. Comparison of 6 DNA extraction methods for isolation of high ... [https://bmcgenomics.biomedcentral.com/articles/10.1186/s12864-023-09537-5]
- 67. Comparison evaluation of bacterial DNA extraction ... [https://www.nature.com/articles/s41598-025-87225-y]
- 68. Advances in human papillomavirus detection for cervical cancer... [https://pubs.rsc.org/en/content/articlelanding/2025/ay/d4ay01921k]
- 69. of boiling Comparison techniques in nucleic... versus magnetic bead [https://virologyj.biomedcentral.com/articles/10.1186/s12985-025-02999-x]
- 70. Automated high throughput DNA isolation for detection of ... [https://www.sciencedirect.com/science/article/abs/pii/S1386653210004920]
- 71. Technical comparison of Human Papillomavirus ... [https://www.dovepress.com/a-technical-comparison-of-human-papillomavirus-genotyping-assays-from--peer-reviewed-fulltext-article-CMAR]
- 72. Acid-activated bentonite for solid-phase nucleic ... [https://www.sciencedirect.com/science/article/abs/pii/S0003267025003228]
- 73. Comparison and Optimization of DNA Extraction Methods ... [https://www.mdpi.com/2036-7503/17/2/30]
- 74. Predicting sequence-specific amplification efficiency in ... [https://www.nature.com/articles/s41467-025-64221-4]
- 75. Real-Time PCR (qPCR) and Whole-Transcriptome NGS [https://www.thermofisher.com/us/en/home/life-science/pcr/real-time-pcr/ngs-comparison.html]
- 76. NGS vs qPCR [https://www.illumina.com/science/technology/next-generation-sequencing/beginners/advantages/ngs-vs-qpcr.html]
- 77. Comparison of commercial systems for extraction of nucleic ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7112907/]
- 78. Top lab trends for 2025 | LabLeaders [https://diagnostics.roche.com/global/en/article-listing/lab-leaders/article/top-lab-trends-2025.html]
- 79. Comparative evaluation of various DNA extraction methods ... [https://bmcbiotechnol.biomedcentral.com/articles/10.1186/s12896-024-00933-7]
- 80. Recent developments and future directions in point-of-care ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11717804/]
- 81. Machine learning in point-of-care testing: innovations, ... [https://www.nature.com/articles/s41467-025-58527-6]